Penicillins are beta-lactam drugs that inhibit the cross-linking of N-acetyl glucosamine (NAG) and N-acetyl muramic acid (NAM) required for the formation of peptidoglycan in bacterial cells. They are generally bacterial cell wall synthesis inhibitors. Penicillin, the first member of the penicillins is a general purpose antibiotic used in clinical medicine even till date. They specifically inhibit the activity of transpeptidases, enzymes that catalyzes the final cross-linking step in the synthesis of peptidoglycan or murein in bacteria (especially Gram positive bacteria). Peptidoglycan or murein gives bacteria their shape and rigidity; and once their synthesis is interfered with, a bacterium loses its structural integrity and becomes susceptible to harmful substances in its environment including antibiotics.
SOURCE OF PENICILLIN
The natural penicillins are derived from moulds such as Penicillium chrysogenum and P. notatum through a fermentation process. Penicillin G (benzyl penicillin) and penicillin V (phenoxymethyl penicillin) are examples of naturally-synthesized penicillins. Ampicillin, oxacillin, methicillin and amoxycillin amongst others are examples of semi-synthesized penicillins (or beta-lactam drugs) produced by chemical modification of the 6-aminopenicillanic acid (produced naturally and by the fermentative activity of moulds especially Penicillium). Addition of side chains to the 6-aminopenicillanic acid leads to the formation of semi-synthetic penicillins as exemplified above. The majority of penicillins and other beta-lactam drugs are now produced by chemical modification processes, and these agents are generally known as semi-synthetic drugs.
STRUCTURE OF PENICILLIN
The general structure of penicillins is known as 6-aminopenicillanic acid (Figure 1). The main component of this structure includes a beta-lactam ring, a thiazolidine ring and a side chain (for the substitution of molecules to derive new semi-synthetic penicillins or variants of the antibiotic such as methicillin and ampicillin amongst others). The beta-lactam ring is the site for beta-lactamase attack; and beta-lactamases are enzymes produced by bacteria, and which interferes with the structural integrity of penicillins and other beta-lactam agents.
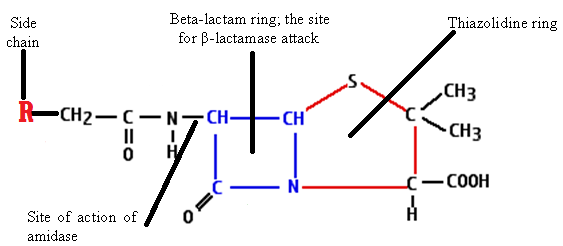
The structural integrity of 6-aminopenicillanic acid is important to the biological function of penicillins- whose main antimicrobial activity is to interfere with cell wall synthesis in the target pathogenic bacteria. Once the beta-lactam ring of 6-aminopenicillanic acid is cleaved by beta-lactamase enzymes (or penicillinase), a new compound known as penicilloic acid (devoid of any antibacterial activity) will be formed.
CLINICAL APPLICATION OF PENICILLIN
Penicillins are general purpose antibacterial drugs, and they have applications in a variety of bacterial-related diseases or infections. Clinically, benzyl penicillin (penicillin G) which is administered parenterally is active against both Gram positive and Gram negative bacteria including Staphylococcus species, Streptococcus species, Neisseria species, Clostridium species, actinomycetes and spirochaetes. Penicillin V is administered orally because it is more resistant to the acidic conditions of the stomach or GIT than penicillin G; and it is active against a variety of Gram positive bacteria such as Streptococcus species and Streptococci species. Since most penicillins (including penicillin G and penicillin V) are clinically ineffective against some Gram negative bacteria (with a complex cell wall than Gram positive bacteria); the therapeutic efficacy of penicillins have been amplified by the chemical modification of their side chains. And this has led to the development of more effective penicillins including amoxicillin, piperacillin, ticarcillin and ampicillin amongst others which have activity against Gram negative organisms (e.g. Klebsiella species, Escherichia coli and Pseudomonas Species).
These later penicillins (e.g. amoxycillin and ticarcillin) are usually co-administered with other non-beta-lactam agents and beta-lactamase inhibitors (e.g. clavulanic acid) so that their therapeutic potential in vivo could be increased. Note: clavulanic acid is not an antibiotic but a beta-lactamase inhibitor; and its main biological function is to help an antibacterial agent (particularly beta-lactams) to conquer bacterial resistance in vivo. Sulbactam and tazobactam are other examples of beta-lactamase inhibitors. Augmentin (or amoxicillin-clavulanic acid) is a combination of amoxicillin and clavulanic acid; and it is semi-synthetic antibiotic used clinically to surmount the problem of resistance in pathogenic bacteria that produces beta-lactamase enzymes which naturally deactivates penicillins and other beta-lactams.
SPECTRUM OF ACTIVITY OF PENICILLIN
The penicillins differ in their spectrum of activity. While some penicillins are more effective against Gram positive bacteria (with a simplified cell wall), others are more effective against Gram negative bacteria (with a cell wall surrounded by an outer membrane). The penetration of the outer membrane (OM) of Gram negative bacteria by some penicillin (e.g. penicillin G) is usually difficult and limited because the OM prevents the entry of the drug to their target site of action- which is the cell wall synthesis machinery of the bacterium. Generally, the penicillins show both narrow spectrum of activity and broad spectrum of activity since they vary in their antibacterial activity which is dependent on the type of drug used and on the target microbe. For example, ticarcillin, ampicillin and carbenicillin show a broader spectrum of activity and can be used absent Gram negative and Gram positive bacteria; but penicillin and methicillin show a narrower spectrum of activity and are mainly active against Gram positive bacteria. Penicillins are bacteriostatic in action since they disrupt the structural integrity of the bacterial cell, thus making them more susceptible to harmful substances including antibiotics.
MECHANISM OR MODE OF ACTION OF PENICILLIN
Penicillins are generally cell wall inhibitors, and drugs in this category (including other beta-lactam agents) inhibit the synthesis of cell wall only in actively growing bacteria. They have no activity on wall-less bacteria (e.g. mycoplasmas). Upon administration, the penicillins specifically bind to the penicillin-binding-proteins (PBPs) on the target bacterial cell. PBPs (e.g. transpeptidases and endopeptidases) are series of receptors found on the cell wall and cell membrane of bacteria; and the main biological activity of the PBPs is to catalyze the cross-linking of the amino acid side chains with those of the glucan backbone of peptidoglycan. A successful cross-linking reaction catalyzed by the transpeptidases result in the formation of a rigid bacterial cell wall; and this whole process is known as transpeptidation reaction. Penicillins bind to transpeptidases, and this prevents PBPs from carrying out its biological function of cell wall synthesis in bacteria. An incomplete cell wall is synthesized, and this leads to the death of the bacterium due to the influx of water and other harmful substances into the high osmotic pressure of the bacterial cell. The penicillins are only active on growing bacteria which synthesize new peptidoglycan; and this makes these agents to be bacteriocidal in action because they prevent the cross-linking reactions of the peptidoglycan which is lacking in non-growing bacteria.
BACTERIAL RESISTANCE TO PENICILLIN
Most penicillin-derivatives are inactivated by penicillinases or beta-lactamase enzymes which makes these drugs to be less-effective for clinical applications. Pathogenic bacteria produce antibiotic-degrading enzymes (e.g. beta-lactamase enzymes) which hydrolyze penicillins by binding to the beta-lactam ring of 6-aminopenicillanic acid, the basic structure of penicillins. Some bacteria mutate and alter their PBPs so that there will be a decreased binding of the antibiotic to the target PBPs. Pathogenic bacteria (especially Gram negative rods) also harbour plasmids that produces antibiotic-degrading enzymes (e.g. extended spectrum beta-lactamases, ESBLs) with a broader hydrolyzing activity on the penicillins and other beta-lactam drugs. Others use the influx-efflux mechanism to stimulate the exclusion of the drug from their internal environment; and this prevents the intracellular accumulation of the antibiotic necessary to exert their injurious activity inside the bacterial cell. Methicillin is a penicillinase-resistant penicillin used clinically to counter the activity of penicillin resistance, but pathogenic bacteria that are resistant to methicillin (i.e. methicillin-resistant Staphylococcus aureus,MRSA) and other penicillins now exist. Oxacillin and nafcillin, though with a narrow-spectrum of activity are used to treat infections in which resistance to methicillin arises.
PHARMACOKINETICS OF PENICILLIN
The absorption of penicillin in the body when administered orally is poor. Complete absorption is mainly achieved when the drug is administered parenterally especially intravenously (IV) or intramuscularly (IM). Penicillins are widely distributed in the body, and they are excreted via the kidney particularly through glomerular filtration and tubular secretion.
SIDE EFFECT/TOXICITY OF PENICILLIN
Penicillins are generally nontoxic antibiotics. They are usually the least toxic of all the antibiotics. Untoward effect resulting from the clinical use of penicillins is usually due to hypersensitivity or allergic reaction to the drug. Mild diarrhea can occur in some patients when penicillin is administered orally. Allergic reaction (which is usually IgE-mediated) due to penicillin usage only occurs in some patients and this can be seen as skin rashes and anaphylaxis in the host taking the drug. Death rarely occurs following the usage of penicillins; and this is usually in those who are allergic to the drug.
References
Ashutosh Kar (2008). Pharmaceutical Microbiology, 1st edition. New Age International Publishers: New Delhi, India.
Axelsen P. H (2002). Essentials of Antimicrobial Pharmacology. Humana Press, Totowa, NJ.
Balfour H. H (1999). Antiviral drugs. N Engl J Med, 340, 1255–1268.
Bean B (1992). Antiviral therapy: current concepts and practices. Clin Microbiol Rev, 5, 146–182.
Beck R.W (2000). A chronology of microbiology in historical context. Washington, D.C.: ASM Press.
Champoux J.J, Neidhardt F.C, Drew W.L and Plorde J.J (2004). Sherris Medical Microbiology: An Introduction to Infectious Diseases. 4th edition. McGraw Hill Companies Inc, USA.
Chemotherapy of microbial diseases. In: Chabner B.A, Brunton L.L, Knollman B.C, eds. Goodman and Gilman’s The Pharmacological Basis of Therapeutics. 12th ed. New York, McGraw-Hill; 2011.
Chung K.T, Stevens Jr., S.E and Ferris D.H (1995). A chronology of events and pioneers of microbiology. SIM News, 45(1):3–13.
Courvalin P, Leclercq R and Rice L.B (2010). Antibiogram. ESKA Publishing, ASM Press, Canada.
Denyer S.P., Hodges N.A and Gorman S.P (2004). Hugo & Russell’s Pharmaceutical Microbiology. 7th ed. Blackwell Publishing Company, USA. Pp.152-172.
Dictionary of Microbiology and Molecular Biology, 3rd Edition. Paul Singleton and Diana Sainsbury. 2006, John Wiley & Sons Ltd. Canada.
Drusano G.L (2007). Pharmacokinetics and pharmacodynamics of antimicrobials. Clin Infect Dis, 45(suppl):89–95.
Engleberg N.C, DiRita V and Dermody T.S (2007). Schaechter’s Mechanisms of Microbial Disease. 4th ed. Lippincott Williams & Wilkins, Philadelphia, USA.
Finch R.G, Greenwood D, Norrby R and Whitley R (2002). Antibiotic and chemotherapy, 8th edition. Churchill Livingstone, London and Edinburg.
Discover more from #1 Microbiology Resource Hub
Subscribe to get the latest posts to your email.


