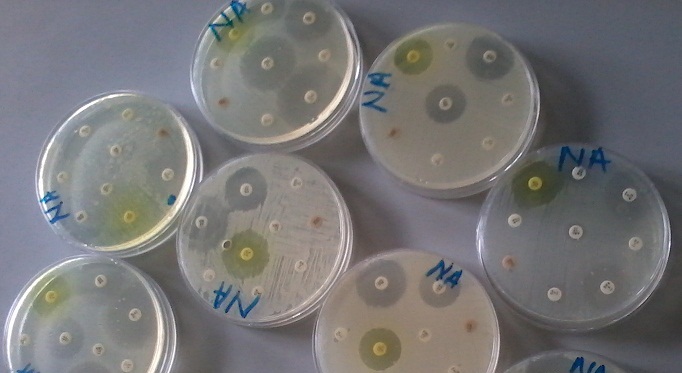
Pharmaceutical microbiology is the branch of microbiology that focuses on all aspects of pharmacy especially as it relates to the manufacture and quality control of pharmaceuticals such as drugs, vaccines, and medical devices. It is an applied branch of microbiology that focuses on the study of microorganisms that are directly or indirectly involved in the manufacture of pharmaceutical products. Pharmaceutical microbiologists ensure that starting raw materials for the manufacture of pharmaceuticals including water are sterile enough and free from any form of contaminating organisms. They carry out series of tests on starting materials for the manufacture of pharmaceuticals as well as test the finished products to ensure their safety and efficacy in treating the ailments they are intended for. Pharmaceutical microbiologists focus heavily on quality control to ensure a supply of life-saving drugs and vaccines that are free from contamination by microorganisms.
Pharmaceutical microbiologists working in a pharmaceutical company are usually incorporated into the Quality Control or assurance department where they help to ensure the quality of finished products as well as that of the raw materials before they are processed in the production area. They also monitor the microbiological quality of air using air monitoring techniques or air samplers to ensure that the air entering the production unit is of good quality and less contaminated.
The functions of a pharmaceutical microbiologist include but not limited to:
- Determination of antimicrobial effectiveness,
- Determination of microbial contamination of an environment or pharmaceutical product,
- Determination of the bioburden of finished products and raw materials,
- Analyzing samples for endotoxins,
- Identifying flora from environmental and/or pharmaceutical monitoring processes in the pharmaceutical industry.
Microbiological applications are tremendously applied in the pharmaceutical industry to produce a wide range of products including hormones, antibiotics, water for injections, and steroids – which are used for the treatment and management of both infectious and non-communicable diseases. Irrespective of the suffering of patients due to infectious diseases caused by pathogenic microorganism, healthcare delivery has tremendously improved worldwide owing to the availability of effective medicines and vaccines with which to treat and prevent these diseases. Pharmaceutical companies around the world are investing heavily in research and development (R&D); and they are also in high demand for pharmaceutical microbiologists due to the relevance of this branch of microbiology in the manufacture of safe, effective and good-quality drugs.
Pharmaceutical microbiology also deals with the controlling of microorganisms that cause spoilage of pharmaceutical products, and this area of microbiology is also keenly interested in harnessing the metabolic activities of microorganisms to develop novel and potent medicines and other pharmaceuticals for the health sector. This branch of microbiology is a burgeoning area in the biological sciences due to its importance to not just the health and pharmaceutical sector, but also the central role that it plays in ensuring the improvement of world health and disease prevention. The production of novel drugs from herbal plants and other natural products are also the subject of pharmaceutical microbiologists.
References
Arora D.R (2004). Quality assurance in microbiology. Indian J Med Microbiol, 22:81-86.
Ashutosh Kar (2008). Pharmaceutical Microbiology, 1st edition. New Age International Publishers: New Delhi, India.
Axelsen P.H (2002). Essentials of antimicrobial pharmacology. Humana Press, Totowa, New Jersey, USA. Al-Jasser A.M (2006). Extended – Spectrum Beta – Lactamases (ESBLs): A Global Problem. Kuwait Medical Journal, 38(3):171-185.
Bisht R., Katiyar A., Singh R and Mittal P (2009). Antibiotic Resistance – A Global Issue of Concern. Asian Journal of Pharmaceutical and Clinical Research, 2 (2):34-39.
Block S.S (2001). Disinfection, sterilization and preservation. 5th edition. Lippincott Williams & Wilkins, Philadelphia and London.
Cars O and Nordberg P (2005). Antibiotic resistance: The faceless threat. International Journal of Risk & Safety in Medicine, 17 (3/4): 103-110.
Carson C.F., Hammer K.A and Riley T.V (2006). Malaleuca alternifolia (Tea Tree) oil: A Review of Antimicrobial and other Medicinal Properties. Clinical Microbiology Review, 19(1):50-62.
Cowan M.M (1999). Plant products as antimicrobial agents. Clinical Microbiology Reviews., 564-582.
Denyer S.P., Hodges N.A and Gorman S.P (2004). Pharmaceutical Microbiology. 7th ed. Blackwell Publishing Company, USA.
Ejikeugwu Chika, Ikegbunam Moses, Ugwu Chigozie, Eze Peter, Iroha Ifeanyichukwu, and Esimone Charles (2013). Phenotypic Detection of Klebsiella pneumoniae Strains – Producing Extended Spectrum β-Lactamase (ESBL) Enzymes. Scholars Academic Journal of Biosciences. 1(1):20-23.
Ejikeugwu Chika, Iroha Ifeanyichukwu, Adikwu Michael and Esimone Charles (2013). Susceptibility and Detection of Extended Spectrum β-Lactamase Enzymes from Otitis Media Pathogens. American Journal of Infectious Diseases. 9(1):24-29.
Ejikeugwu Chika, Iroha Ifeanyichukwu, Adikwu Michael and Esimone Charles (2013). Susceptibility and Detection of Extended Spectrum β-Lactamase Enzymes from Otitis Media Pathogens. American Journal of Infectious Diseases. 9(1):24-29.
Ejikeugwu Chika, Ugwu C.M., Ikegbunam N.M, Araka C.O., Iroha I.R., Adikwu M.U and Esimone C.O (2012). Evaluation of antibacterial activity of the leave extracts of Buchholzia coriacea. Asian Journal of Pharmaceutical and Biological Research. 2(4):204-208.
Ejikeugwu Chika, Ugwu Malachy, Iroha Ifeanyichukwu, Gugu Thaddeus, Duru Carissa, Eze Peter, and Esimone Charles (2013). Detection and antimicrobial susceptibility of some Gram negative bacteria producing carbapenemases and extended spectrum beta lactamases. International Journal of Microbiology and Immunology Research, 2(6):064-069.
Ejikeugwu Chika, Umeokoli Blessing, Iroha Ifeanyichukwu, Ugwu Malachy, Esimone Charles (2015). Phytochemical and Antibacterial Screening of Crude Extracts from Leaves of Wonderful Kola. American Journal of Life Sciences. Special Issue: Microbiology Research, 3(2):5-8.
Ejikeugwu P.C., Ugwu C.M., Araka C.O., Gugu T.H., Iroha I.R., Adikwu M.U and Esimone C.O (2012). Imipenem and Meropenem resistance amongst ESBL producing Escherichia coli and Klebsiella pneumoniae clinical isolates. International Research Journal of Microbiology. 3(10):339-344.
Finch R.G, Greenwood D, Norrby R and Whitley R (2002). Antibiotic and chemotherapy, 8th edition. Churchill Livingstone, London and Edinburg.
Joslyn, L. J. (2000). Sterilization by Heat. In S. S. Block (Ed.), Disinfection, Sterilization, and Preservation (5th ed., pp. 695-728). Philadelphia, USA: Lippincott Williams and Wilkins.
Lai P.K and Roy J (2004). Antimicrobial and chemopreventive properties of herbs and spices. Curr. Med. Chem, 11 (11): 1451–1460.
Livermore D.M (2004). The need for new antibiotics. Clinical Microbiology & Infection, 4(10): 1-9.
Mascaretti O.A (2003). Bacteria versus antibacterial agents: An integrated approach. Washington: ASM Press.
Nally J.D (Ed.) (2007). Good manufacturing practices for pharmaceuticals. Sixth edition. Informa Healthcare USA, Inc, New York.
Discover more from #1 Microbiology Resource Hub
Subscribe to get the latest posts to your email.