Antimicrobial susceptibility tests are used to determine which specific antibiotics or antimicrobial agents a particular pathogenic bacterium or fungus is sensitive to; and susceptibility tests can guide the physician in drug choice and dosage especially for difficult-to-treat infections. The results of antimicrobial susceptibility tests or studies are commonly reported as the minimal inhibitory concentration (MIC), which is the lowest concentration of drug that inhibits the growth of the organism; and such reports typically contain a quantitative result in µg/mL and a qualitative interpretation.
The unit for MIC is µg/mL. The interpretation usually categorizes each result as either Susceptible (S), Intermediate (I), or Resistant (R). In some cases, the antimicrobial susceptibility test result can also be interpreted as “no interpretation” (NI) – which implies that the MIC of the antimicrobial could not be determined at the concentrations of the drug used. It is noteworthy that most in vitro susceptibility testing was performed by the disk diffusion method – in which the size of the growth-free zone (known as the inhibition zone diameter, IZD of the test drug)determined whether the test bacterium was considered to be susceptible, resistant or intermediate to a particular antibiotic being tested.
The Kirby-Bauer disk diffusion technique is one of such techniques used in determining by in vitro technique the susceptibility of a particular organism to a given antimicrobial agent. The Kirby-Bauer disk diffusion technique is a test which uses antibiotic-impregnated in a paper disk to test whether a bacterium is affected by antibiotics. In the Kirby-Bauer disk diffusion technique, the test bacterium is aseptically swabbed uniformly across a culture plate; and a filter-paper disk (impregnated with the antibiotic to be tested) is then placed on the surface of the agar. The antibiotic diffuses from the filter paper into the agar.
If the antibiotic is effective against the test bacterium at a certain concentration, no colonies of the test organism will grow around the filter-paper disk impregnated with the test drug. This area where no bacterial growth is observed around the drug-impregnated filter paper disk is known as the zone of inhibition or inhibition zone diameter (IZD). The size of this zone depends on how effective the antibiotic is at stopping the growth of the bacterium. A stronger antibiotic will create a larger zone, because a lower concentration of the antibiotic is enough to stop growth.
The Kirby-Bauer disk diffusion technique does not give the clinical concentration of drug required to treat a particular sickness – as is applicable with the determination of the drug’s MIC. Generally, MIC determination is mainly used in the comparative testing of novel antimicrobial agents or antibiotics. And in the hospital or clinical microbiology laboratories, MIC determination is used to establish the susceptibility of pathogenic organisms that give equivocal or indistinct results in the Kirby-Bauer disk diffusion technique.
MIC can also be used to determine the susceptibility of pathogens whose antimicrobial susceptibility patterns or antibiogram cannot be evaluated by the disk diffusion method. When a more accurate antimicrobial susceptibility is often needed for the best clinical management of a particular patient, MIC determination is usually the best option in such scenarios. Minimum inhibitory concentration (MIC) is the lowest concentration of an antimicrobial that will inhibit the visible growth of a microorganism after overnight incubation. It is the lowest concentration of antibiotic at which the growth of a pathogenic bacterium is inhibited.
MIC is different from the minimum bactericidal concentration (MBC) – which is the lowest concentration of antibiotic at which a pathogenic bacterium is killed. MBC is the lowest concentration of antimicrobial agent that will prevent the growth of an organism after subculture onto antibiotic-free culture media. The MIC values help the microbiologist and/or physician to ascertain the actual drug concentration that will be clinically relevant to inhibit the growth of the test organism in vivo.
MIC helps to measure more exactly the exact concentration of a drug that is required to inhibit the growth of standard inoculum of an organism under some defined laboratory conditions. MIC values are also used by diagnostic laboratories and hospitals mainly to confirm resistance in resistant bacteria pathogens. However, this important antimicrobial susceptibility study (i.e. MIC) is most often used as a research tool to determine the in vitro activity of new antimicrobial agents. Data from such research studies to develop novel antimicrobials have been used to determine the MIC breakpoints – which now serve as a guide or standards in measuring or comparing the MIC values of different antimicrobial agents.
The MIC value of an antimicrobial can be determined by culturing microorganisms in liquid media or on culture tubes of liquid growth medium. A lower MIC value indicates that less drug is required for inhibiting growth of the organism. Thus the antimicrobial with the lower MIC values are referred to as the more effective antimicrobial agent tested. MIC values help in improving the treatment outcomes for patients and it also help to prevent the evolution and spread of drug-resistant microbial strains by identifying appropriate drugs and their effective concentrations for a particular treatment especially on a patient-to-patient basis.
Physicians use MIC values to choose which antibiotics and/or antimicrobial agent is best suited to administer to a particular sick patient; and the MIC values also help the clinician to known the actual concentration or effective dose of antibiotic to administer. This practice is important in clinical medicine and clinical research because bacterial populations exposed to an insufficient concentration of a particular antimicrobial agent or to a broad-spectrum antibiotic can evolve resistance to these antibiotics; and thus become ineffective for treatment.
There are several commercial methods available to experimentally measure the MIC values of antimicrobial agents. Dilution methods are used to determine the MIC value of a given antimicrobial agent. Agar well dilution technique and broth dilution technique are two important dilution methods used in determining the MIC value of antimicrobial agents. The aim of broth and agar dilution methods is to determine the lowest concentration of the assayed antimicrobial agent (minimal inhibitory concentration, MIC) that, under defined test conditions, inhibits the visible growth of the bacterium being investigated.
Pathogenic microorganisms can be tested for their ability to produce visible growth (turbidity) on a series of agar plates (agar dilution method), in tubes with broth (broth dilution method), or in microtiter plate wells of broth (broth microdilution method) containing varying dilutions of an antimicrobial agent. In dilution test methods such as the agar well and broth dilution techniques, the test microorganisms are tested for their ability to produce visible growth in micro-titration plate wells of broth (broth microdilution) containing serial dilutions of the test antimicrobial agents.
In order to identify the MIC of an antimicrobial through the broth dilution method, identical doses of the test bacteria are cultured in wells or tubes of liquid media containing progressively lower concentrations of the test antimicrobial agent. The minimum inhibitory concentration of the antibiotic is between the concentrations of the last well or tube in which no bacteria grew and the next lower dose, which allowed bacterial growth. The lowest concentration of the test antimicrobial agent or antibiotic that will inhibit the visible growth of the test microorganism is known as the MIC of the drug (Figure 1).
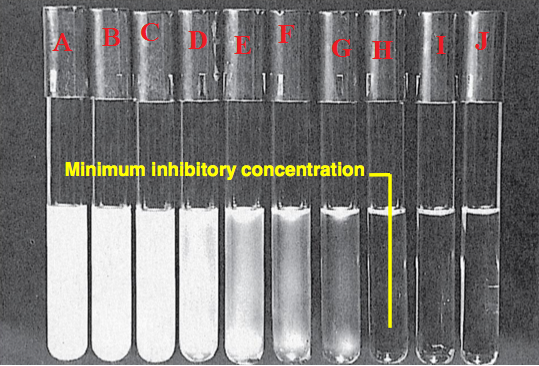
MODUS OPERANDI FOR PERFORMING MIC IN THE LABORATORY (TWO FOLD SERIAL DILUTION TECHNIQUE)
- Set up 5 test tubes or more in a test tube rack and label them tube A, tube B, tube C, tube D and tube E as the case may be (Figure 1). Two additional test tubes should also be set up for the positive and negative control. Negative control tube contains the bacterial culture and the broth and remains turbid. Positive control tube is clear and contains only the broth and the test drug. Mueller-Hinton (MH) broth is a general purpose medium which is used to perform antimicrobial susceptibility studies in the microbiology laboratory.
- Add 0.5 ml of Mueller-Hinton (MH) broth to each of the tubes (labeled A-E) using a sterile pipette.
- Add 0.5 ml of the test drug (from a stock concentration of 250 mg/ml) to the first test tube (i.e. tube A). Mix the solution properly. Note: upon dilution tubes A, B, C, D and E will be 25 mg/ml, 12.5 mg/ml, 6.25 mg/ml, 3.125 mg/ml and 1.563 mg/ml.
- Transfer 0.5 ml of the diluted antibiotic solution from tube A to tube B.
- Repeat this process until all the tubes have been covered. Dispense or discard 0.5 ml of the diluted antibiotic solution from the last test tube (i.e. tube E).
- Transfer 0.5 ml of bacterial suspension (adjusted to 0.5 McFarland turbidity standards) to each of the tubes (labeled A-E). Mix the content of each tube properly.
- Incubate the tubes at 37oC for 24 hr or overnight.
- Check each of the tubes for turbidity or cloudiness after incubation. Ensure not to shake the tubes when doing so in order to get a non-biased reading. Check out bacterial growth in the tubes (as indicated by the presence of cloudiness or turbidity).
- The tube without cloudiness or turbidity is the MIC of the antibiotic for the test bacteria. Absence of cloudiness is indicative of the inhibitory effect of the test agent against the bacteria in broth culture. Presence of turbidity in all the tubes should be reported as “not determined (ND)”, and this implies that MIC was not determined at the concentrations of the drug used.
- Minimum bactericidal concentration (MBC) is the lowest concentration of the drug that can kill the test pathogenic bacteria. MBC is the lowest concentration of a drug that will kill a pathogenic bacterium. It is determined from the MIC by subculturing the content of the tubes that shows no bacterial growth onto a fresh agar plate devoid of antibiotic. MBC is evaluated as the lowest drug concentration from which the test pathogenic bacteria do not grow after an overnight incubation.
References
Arora D.R (2004). Quality assurance in microbiology. Indian J Med Microbiol, 22:81-86.
Ashutosh Kar (2008). Pharmaceutical Microbiology, 1st edition. New Age International Publishers: New Delhi, India.
Barenfanger J, Drakel C and Kacich (1999). Clinical and Financial Benefits of Rapid Bacterial Identification and Antimicrobial Susceptibility Testing. Journal of Clinical Microbiology, 37(5):1415-1418.
Denyer S.P., Hodges N.A and Gorman S.P (2004). Pharmaceutical Microbiology. 7th ed. Blackwell Publishing Company, USA.
Doern G, Brueggemannn A.B, Perla R, Daly D, Halkias D, Jones R.N, Saubolle M.A (1997). Multicenter laboratory evaluation of the bioMerieux Vitek antimicrobial susceptibility testing system with 11 antimicrobial agents versus members of the family Enterobacteriaceae and Pseudomonas aeruginosa. J Clin Microbiol, 35:2115–2119.
Doern G, Vautour R, Gaudet M, Levy B (1994). Clinical impact of rapid in vitro susceptibility testing and bacterial identification. J Clin Microbiol, 32:1757–1762.
Doern G.V (1995). Susceptibility tests of fastidious bacteria. Manual of Clinical Microbiology, 6th edition, Murray P.R, Baron E.J, Pfaller M.A, Tenover F.C, Yolken R, American Society for Microbiology, Washington DC, Pp. 1342-1349.
Funke G, Monnet D, deBernardis C, von Graevenitz A, Freney J (1998). Evaluation of the VITEK 2 system for rapid identification of medically relevant Gram-negative rods. J Clin Microbiol, 36:1948–1952.
Garcia L.S (2010). Clinical Microbiology Procedures Handbook. Third edition. American Society of Microbiology Press, USA.
Hart C.A (1998). Antibiotic Resistance: an increasing problem? BMJ, 316:1255-1256.
Livermore D.M, Winstanley T.B, Shannon K.P (2001). Interpretative reading: recognizing the unusual and inferring resistance mechanisms from resistance phenotypes. J Antimicrob Chemother, 48 Suppl 1:87-102.
Madigan M.T., Martinko J.M., Dunlap P.V and Clark D.P (2009). Brock Biology of Microorganisms, 12th edition. Pearson Benjamin Cummings Inc, USA.
Mahon C. R, Lehman D.C and Manuselis G (2011). Textbook of Diagnostic Microbiology. Fourth edition. Saunders Publishers, USA.
National Committee for Clinical Laboratory Standards. Performance Standards for antimicrobial susceptibility testing. 8th Informational Supplement. M100 S12. National Committee for Clinical Laboratory Standards, 2002. Villanova, Pa.
Washington J.A (1993). Rapid antimicrobial susceptibility testing: technical and clinical considerations. Clin Microbiol Newsl, 15:153–155.
Discover more from #1 Microbiology Resource Hub
Subscribe to get the latest posts to your email.