Ciprofloxacin is a fluoroquinolone antibiotic that is derived from the earlier quinolones. Nalidixic acid is a typical example of a quinolone; and both the quinolones and fluoroquinolones are bacterial DNA replication inhibitors. But antibiotics in the fluoroquinolone family have broader activity than the quinolones. Other examples of fluoroquinolones include ofloxacin, norfloxacin, sparfloxacin, lomefloxacin and levofloxacin. Fluoroquinolones are fluorinated quinolones or newer quinolone antibiotics, and they have superior antibacterial activity than the quinolones (e.g. nalidixic acid).
SOURCES OF CIPROFLOXACIN
Ciprofloxacin, a fluoroquinolone is a synthetic antibiotic that is produced by chemical modification of the quinolone structure. Both the fluoroquinolones and quinolones are synthetic chemotherapeutic antibacterial agents; and they are not produced naturally by microbes.
STRUCTURE
The structure of quinolones from which fluoroquinolones (e.g. ciprofloxacin) are synthetically derived from is a nucleus of two fused 6-membered rings (Figure 1) that is chemically substituted with a fluorine molecule to form fluoroquinolones. The incorporation of fluorine molecules into the quinolone nucleus gives ciprofloxacin and other fluoroquinolones an enhanced antibacterial activity; and this is why they are more preferred than the quinolones (e.g. nalidixic acid) in clinical medicine. The chemical structures of some fluoroquinolones are shown in Figure 2.
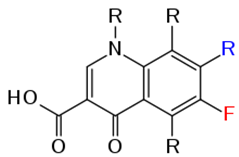
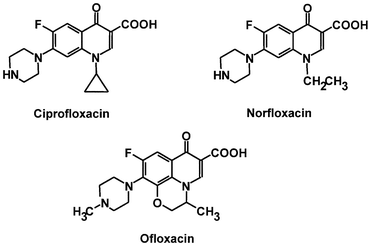
CLINICAL APPLICATION OF CIPROFLOXACIN
Ciprofloxacin is used to treat bacterial infections caused by Gram negative and Gram positive bacteria; and they are often used in synergy with other antibacterial agents. The fluoroquinolones (including ciprofloxacin) is used to treat anthrax (caused by B. anthracis). And they are used clinically to treat UTIs, respiratory tract infections, skin infections, and infections caused by anaerobic bacteria and Chlamydia. The quinolones (e.g. nalidixic acid) are basically used to treat UTIs; and they are mainly against Gram-negative bacteria.
SPECTRUM OF ACTIVITY OF CIPROFLOXACIN
Ciprofloxacin and other fluoroquinolones have a broad spectrum of activity and they are bacteriocidal in action. They have activity against both Gram-positive bacteria and Gram-negative bacteria. Quinolones are effective for the treatment of UTIs especially those caused by bacteria in the family Enterobacteriaceae; and they are mainly active against some Gram-negative bacteria, and with little or no antibacterial activity against Gram positive bacteria.
MECHANISM OR MODE OF ACTION OF CIPROFLOXACIN
Ciprofloxacin and other fluoroquinolones as well as the quinolones are generally DNA synthesis inhibitors (Figure 3). They mainly bind to the DNA gyrase enzyme (or topoisomerase) during bacterial DNA replication; and this binding prevents the enzyme (DNA gyrase or topoisomerase IV) from carrying out its biological function of cutting, repairing and coiling DNA molecules during bacterial DNA replication. Once ciprofloxacin binds to the topoisomerase enzyme, the function of DNA gyrase in DNA replication will be inhibited. The target pathogenic bacteria eventually die because the inhibition of DNA synthesis in bacteria blocks cell division, and this obstructs other important cellular activities of the organism.
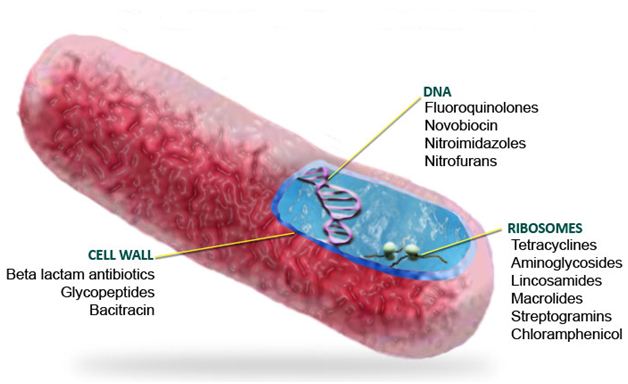
BACTERIAL RESISTANCE TO CIPROFLOXACIN
Bacterial resistance to the fluoroquinolones and quinolones is usually due to mutation in the chromosome of the organism which makes them to be less-susceptible to the drug. Mutation in the active site or binding site of DNA gyrase enzymes prevents fluoroquinolones or quinolones to bind, and this can make a bacterium to be resistant to the antibiotic.
PHARMACOKINETICS OF CIPROFLOXACIN
Ciprofloxacin and other fluoroquinolones are well distributed in the body after oral distribution; and they are excreted in urine and bile through the kidney.
SIDE EFFECT/TOXICITY OF CIPROFLOXACIN
Fluoroquinolones and quinolones are contraindicated for pregnant women and infants because the antibiotic damages growing bones and cartilages. Nausea, vomiting, headache and dizziness are some of the mild untoward effects associated with the clinical use of the fluoroquinolones.
References
Ashutosh Kar (2008). Pharmaceutical Microbiology, 1st edition. New Age International Publishers: New Delhi, India.
Axelsen P. H (2002). Essentials of Antimicrobial Pharmacology. Humana Press, Totowa, NJ.
Balfour H. H (1999). Antiviral drugs. N Engl J Med, 340, 1255–1268.
Bean B (1992). Antiviral therapy: current concepts and practices. Clin Microbiol Rev, 5, 146–182.
Beck R.W (2000). A chronology of microbiology in historical context. Washington, D.C.: ASM Press.
Champoux J.J, Neidhardt F.C, Drew W.L and Plorde J.J (2004). Sherris Medical Microbiology: An Introduction to Infectious Diseases. 4th edition. McGraw Hill Companies Inc, USA.
Chemotherapy of microbial diseases. In: Chabner B.A, Brunton L.L, Knollman B.C, eds. Goodman and Gilman’s The Pharmacological Basis of Therapeutics. 12th ed. New York, McGraw-Hill; 2011.
Chung K.T, Stevens Jr., S.E and Ferris D.H (1995). A chronology of events and pioneers of microbiology. SIM News, 45(1):3–13.
Courvalin P, Leclercq R and Rice L.B (2010). Antibiogram. ESKA Publishing, ASM Press, Canada.
Denyer S.P., Hodges N.A and Gorman S.P (2004). Hugo & Russell’s Pharmaceutical Microbiology. 7th ed. Blackwell Publishing Company, USA. Pp.152-172.
Dictionary of Microbiology and Molecular Biology, 3rd Edition. Paul Singleton and Diana Sainsbury. 2006, John Wiley & Sons Ltd. Canada.
Drusano G.L (2007). Pharmacokinetics and pharmacodynamics of antimicrobials. Clin Infect Dis, 45(suppl):89–95.
Engleberg N.C, DiRita V and Dermody T.S (2007). Schaechter’s Mechanisms of Microbial Disease. 4th ed. Lippincott Williams & Wilkins, Philadelphia, USA.
Finch R.G, Greenwood D, Norrby R and Whitley R (2002). Antibiotic and chemotherapy, 8th edition. Churchill Livingstone, London and Edinburg.
Ghannoum MA, Rice LB (1999). Antifungal agents: Mode of action, mechanisms of resistance, and correlation of these mechanisms with bacterial resistance. Clin Microbiol Rev, 12:501–517.
Gillespie S.H and Bamford K.B (2012). Medical Microbiology and Infection at a glance. 4th edition. Wiley-Blackwell Publishers, UK.
Gordon Y. J, Romanowski E.G and McDermitt A M (2005). A review of antimicrobial peptides and their therapeutic potential as anti-infective drugs. Current Eye Research, 30(7): 505-515.
Hardman JG, Limbird LE, eds. Goodman and Gilman’s The Pharmacological Basis of Therapeutics. 10th ed. New York: McGraw-Hill; 2001.
Joslyn, L. J. (2000). Sterilization by Heat. In S. S. Block (Ed.), Disinfection, Sterilization, and Preservation (5th ed., pp. 695-728). Philadelphia, USA: Lippincott Williams and Wilkins.
Katzung, B. G. (2003). Basic and Clinical Pharmacology (9th ed.). NY, US, Lange.
Kontoyiannis D.P and Lewis R.E (2002). Antifungal drug resistance of pathogenic fungi. Lancet. 359:1135–1144.
Discover more from #1 Microbiology Resource Hub
Subscribe to get the latest posts to your email.


