The determination of the antimicrobial susceptibility of a given pathogen to a particular antimicrobial agent can be performed or undertaken by one of two basic methods, viz:
- The Disk Diffusion Method.
- The Dilution Method.
Agar Disk Diffusion Method
Agar disk diffusion technique of antimicrobial susceptibility testing provides a basis for the classification of a pathogenic strain as susceptible, intermediate or resistant to a drug or an array of drugs depending on the zone of inhibition caused by the agent on the growth of the microbe being investigated. The disk diffusion method is one of the more commonly & most widely used methods of antimicrobial drug susceptibility testing in the microbiology laboratory. Agar disk diffusion techniques detect the susceptibility and resistance of microbial cells to antimicrobial agents qualitatively.
It is often used when a rapidly growing aerobic or facultative pathogen like Staphylococcus or Pseudomonas amongst others is being tested. The disk diffusion method is often used in order to save time and media, and its technique is a very simple one. They are used routinely by most clinical microbiology laboratories to all over the world to carry out their antimicrobial drug susceptibility testing.
When an antibiotic impregnated disk or a filter paper disk containing a given amount of a drug is placed on the surface of a solid agar plate previously or already inoculated with the test bacterium, the antibiotic impregnated disk picks up moisture and the antibiotic diffuses radially outward through the agar, producing an antibiotic concentration gradient after an overnight incubation at 37oC.
The diameter of the clear zone (s) of inhibition seen around the antibiotic impregnated disk is taken as a measure of the inhibitory power of the drug against the particular test bacterium. The antibiotic is present at high concentrations near the disk and this affects only susceptible microorganisms, while resistant organisms will grow up to the disk. The clear zone is measured using a meter rule, and compared with standards as per CLSI criteria in order to determine whether the test pathogen is intermediate, resistant, or susceptible to the antimicrobial agent under study.
The zone size measured cannot be used to compare directly the effectiveness of an antimicrobial agent, though it can give a little clue to how much a given drug can impact an organism in vitro. Thus, interpretation of disk diffusion test results must be done with utmost caution. The Kirby – Bauer disk diffusion method is a type of disk diffusion method that is currently used today especially in most clinical microbiology laboratories.
PROCEDURE FOR CARRYING OUT THE KIRBY-BAUER DISK DIFFUSION METHOD
- Pick or touch about 2-3 colonies of the test bacterium on an agar using a sterilized inoculating loop.
- Inoculate it in a tube of nutrient broth.
- Incubate the nutrient broth tube in the incubator for few hours (3 – 4hrs) at 37oC until it becomes slightly turbid.
- Bring out the tube from the incubator and compare or match it to a turbidity standard (in this case, 0.5 McFarland turbidity standards).
- Dilute the content of the tube using normal saline when necessary so as to match up with the turbidity standard.
- Dip a sterile cotton swab stick into the nutrient broth tube containing the test bacterium.
- Rotate the cotton swab stick firmly against the inside wall of the tube in order to remove excess inoculums.
- Streak the entire surface of a Mueller Hinton (MH) agar plate with swab stick.
- Allow the plates for about 10 minutes with lid in place in order for the inoculums to dry and diffuse into the agar medium.
- Aseptically place the antibiotic disc (s) on the inoculated MH agar plate.
- Incubate the plates in the incubator at 37oC overnight.
- Examine the plate (s) and measure the zones of inhibitions produced.
Note: Test isolates for susceptibility studies can also be inoculated in normal saline other than nutrient broth. Add 2-3 colonies of the test bacterium to the normal saline in a sterile test tube and mix. Compare tube with another tube containing the 0.5 McFarland turbidity standards. If the bacterial suspension does not appear to be of the same density as the 0.5 McFarland turbidity standards, the turbidity of the test organism can be adjusted to meet that of the standard by adding sterile saline or more bacterial growth depending on the turbidity of the standard.
Dilution Susceptibility Method
The dilution susceptibility method is usually performed by detecting the growth of microbial cells in broth or agar cultures that contains a defined concentration of an antimicrobial agent. It is used to measure the minimum inhibitory concentration (MIC) of antibiotic that will inhibit the growth of a bacterium and the minimum bactericidal concentration (MBC) as well. MBC is the lowest concentration of antimicrobial agent required to kill bacteria.
MBC can also be used interchangeably with minimum lethal concentration. Unlike the agar diffusion technique, the dilution susceptibility tests detect the susceptibility of microbial cells to antimicrobial agents in a quantitative approach since they measure the growth of pathogenic microorganisms when subjected to different dilutions of drugs in a series of tubes. There are many ways to measure the MIC of a drug against a particular microorganism, including:
- Broth dilution.
- Agar dilution.
- E-test method.
Broth dilution tests
Broth dilution tests are used to test the antimicrobial susceptibility profile of pathogenic bacteria in broth culture medium that contain defined concentrations of a particular test drug. The test drug is usually prepared in two-fold serial dilutions in well labeled test tubes. In the broth dilution antimicrobial susceptibility technique, test bacteria are grown in liquid or broth culture medium that supports the growth of the organism and in which defined antibiotic concentrations are seeded.
Bacterial growth in the medium is defined or evaluated by the presence or absence of turbidity (i.e. cloudiness) in the broth culture. The first tube that shows no bacterial growth (as indicated by the absence of turbidity) is taken as the MIC of the drug. Broth dilution susceptibility studies like the agar dilution tests as shall be seen later in this section are very cumbersome to perform. Several tubes are usually needed to evaluate the susceptibility of a test bacterium by the broth dilution method.
However, the advent of prepared broth dilution series for different drugs in micro dilution plates have significantly enhanced and simplified this technique for drug susceptibility testing in the microbiology laboratory. A micro dilution broth test allows a quantitative result to be reported, indicating the amount of a given drug necessary to inhibit or kill the tested microorganism. Nevertheless, the broth dilution test is not routinely used for evaluating the susceptibility profile of antimicrobial agents.
Agar dilution susceptibility tests
Agar dilution susceptibility tests which is also similar to the broth dilution test are performed on agar plates (prepared according to the manufacturers instruction) using defined concentrations of a pure test drug in the powdered form. Pathogenic bacteria are grown or cultured on solid culture media for agar dilution test unlike in broth dilution technique where the test bacterium is grown in broth or liquid medium.
In agar dilution tests, antimicrobial agents (e.g. antibiotics) are tested at different serial dilutions, and each of the agar plate contain one particular concentration of the test drug. The drug dilutions are aseptically seeded into the agar prior to or during plate pouring before microbial inoculation. After incubation, the agar plates are observed for inhibition of microbial growth. The drug dilution in the agar plate that shows no bacterial growth is recorded as the MIC of the drug against the organism.
They are usually cumbersome to perform. The antibiotic solution used to perform agar dilution is prepared in double fold or two-fold serial dilutions. Thus, their use is often limited to special circumstances like carrying out a research work to determine the MBC or MIC of a given antimicrobial agent. It involves obtaining a standard antibiotic powder from a reputable pharmaceutical company, preparation of antibiotic stock solutions, preparation of antibiotic dilution range, and preparation of agar dilution range.
E-test
E-test is used to determine the MIC of a given antimicrobial agent on a given pathogen. It is more or less another disk diffusion technique used for the evaluation of the susceptibility profile of pathogenic bacteria in the microbiology laboratory. The E-test (which is both a diffusion and dilution technique) is a special plastic test strips that has already been impregnated with antibiotics. Each E-test strip contains a gradient of an antibiotic and is labeled with a scale of MIC values.
It is one of the easiest and most convenient methods of determining the MIC of a particular drug. The lowest concentration in the strip lies at the center of an already inoculated agar plate. Briefly, when the E-test strip is aseptically placed on agar plates previously swabbed or inoculated with the pathogenic test bacteria and incubated at the optimum temperature, the antibiotic impregnated in the strip diffuses in a gradient continuous pattern (Figure 1).
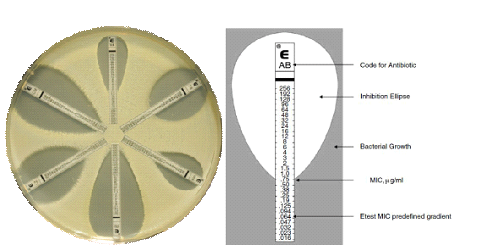
Source: http://www.biomerieux-diagnostics.com/etest
After incubation, an elliptical zone of inhibition appears around the strip, and the point on the E-strip at which the growth inhibition zones intersects is determined as the MIC of the drug contained in the strip. The E-test strip technique gives a fast and reliable method of evaluating the susceptibility profile as well as the MIC of a particular drug against pathogenic bacteria in the microbiology laboratory.
References
Arora D.R (2004). Quality assurance in microbiology. Indian J Med Microbiol, 22:81-86.
Ashutosh Kar (2008). Pharmaceutical Microbiology, 1st edition. New Age International Publishers: New Delhi, India.
Barenfanger J, Drakel C and Kacich (1999). Clinical and Financial Benefits of Rapid Bacterial Identification and Antimicrobial Susceptibility Testing. Journal of Clinical Microbiology, 37(5):1415-1418.
Denyer S.P., Hodges N.A and Gorman S.P (2004). Pharmaceutical Microbiology. 7th ed. Blackwell Publishing Company, USA.
Doern G, Brueggemannn A.B, Perla R, Daly D, Halkias D, Jones R.N, Saubolle M.A (1997). Multicenter laboratory evaluation of the bioMerieux Vitek antimicrobial susceptibility testing system with 11 antimicrobial agents versus members of the family Enterobacteriaceae and Pseudomonas aeruginosa. J Clin Microbiol, 35:2115–2119.
Doern G, Vautour R, Gaudet M, Levy B (1994). Clinical impact of rapid in vitro susceptibility testing and bacterial identification. J Clin Microbiol, 32:1757–1762.
Doern G.V (1995). Susceptibility tests of fastidious bacteria. Manual of Clinical Microbiology, 6th edition, Murray P.R, Baron E.J, Pfaller M.A, Tenover F.C, Yolken R, American Society for Microbiology, Washington DC, Pp. 1342-1349.
Funke G, Monnet D, deBernardis C, von Graevenitz A, Freney J (1998). Evaluation of the VITEK 2 system for rapid identification of medically relevant Gram-negative rods. J Clin Microbiol, 36:1948–1952.
Garcia L.S (2010). Clinical Microbiology Procedures Handbook. Third edition. American Society of Microbiology Press, USA.
Hart C.A (1998). Antibiotic Resistance: an increasing problem? BMJ, 316:1255-1256.
Livermore D.M, Winstanley T.B, Shannon K.P (2001). Interpretative reading: recognizing the unusual and inferring resistance mechanisms from resistance phenotypes. J Antimicrob Chemother, 48 Suppl 1:87-102.
Madigan M.T., Martinko J.M., Dunlap P.V and Clark D.P (2009). Brock Biology of Microorganisms, 12th edition. Pearson Benjamin Cummings Inc, USA.
Mahon C. R, Lehman D.C and Manuselis G (2011). Textbook of Diagnostic Microbiology. Fourth edition. Saunders Publishers, USA.
National Committee for Clinical Laboratory Standards. Performance Standards for antimicrobial susceptibility testing. 8th Informational Supplement. M100 S12. National Committee for Clinical Laboratory Standards, 2002. Villanova, Pa.
Washington J.A (1993). Rapid antimicrobial susceptibility testing: technical and clinical considerations. Clin Microbiol Newsl, 15:153–155.
Discover more from #1 Microbiology Resource Hub
Subscribe to get the latest posts to your email.


