Dracunculiasis is a crippling parasitic disease that is caused by a tissue nematode (roundworm) which is usually found in the subcutaneous tissue of infected humans. It is also known as guinea worm ulcer disease because the disease causes ulcer or boil on the skin of infected human hosts. The roundworm starts to emerge from the skin of the affected human host, usually on the lower limb few months after initial infection.
Dracunculiasis is a predominant protozoal disease in rural poor communities in most parts of the world particularly in Africa where access to good drinking water is still pitiable. The disease was endemic in some parts of Asia such as India and Pakistan, but has since been eradicated from these countries.
Though concerted global efforts are ongoing in eradicating guinea worm disease (GWD) from the face of the earth, the disease is currently present in four African countries including Chad, Ethiopia, Mali, and South Sudan where its containment is also being pursued. Dracunculiasis is caused by the tissue nematode Dracunculus medinensis. D. medinensis, a type of roundworm has both male (which measures about 1-4 cm) and female worms (which measure about 50-100 cm in length).
Type and morphology of the organism
D. medinensis exists in larval forms which measures about 500-700 µm in length. The larva has a long and pointed tail, and its anterior end is usually rounded. Tail of D. medinensis larva can be seen to be coiling and uncoiling under the microscope.
Vector, reservoir and habitat of the organism
Copepods (tiny water fleas) are the most important vectors of D. medinensis. Other intermediate hosts or reservoirs of the parasite include other related freshwater crustaceans such as Cyclops. The best habitat of D. medinensis larva is standing freshwater bodies such as ponds. Streams and rivers may also harbour the vectors of the parasite and help to transmit D. medinensis larva to human hosts.
Clinical signs and symptoms of dracunculiasis
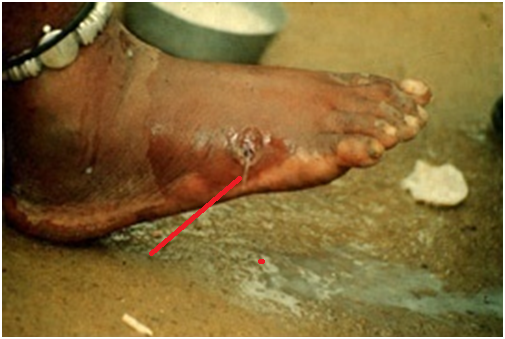
Dracunculiasis causes a localized infection, and the appearance of a blister or sore on the skin which increases in size is typically the first clinical sign of GWD. The clinical manifestation of GWD usually starts to become obvious about one year after infection. There is usually pain, fever and swelling at the site of the skin where the worm exits the body.
Vomiting, gastrointestinal upset, urticaria (i.e. allergic reactions to injections particularly from plants and food) and nausea (feeling of vomiting) are other accompanying symptoms of GWD. Female worms come out of the ulcerative legs and feet of the affected human host, but the protruding worm can also emerge from other parts of the body.
A secondary bacterial infection can ensue from the wound caused by the protruding female worms, and this can incapacitate the affected individual and prevent him/her from taking part in any meaningful economic activity.
The protruding female worm which emerges in about 4 days from the swollen sore appears as a white filament, and it is removed by wrapping it around a stick. Immersion of the swollen leg or feet in cold water relieves the pain caused by the sore and it also aids the extraction of the worm from the body.
Pathogenesis of dracunculiasis
A true case of GWD is often recognized by the protrusion of a white, threadlike worm that emanate from an ulcer on the skin (usually legs or feet) of persons infected with Dracunculus medinensis.Human infection with D. medinensis is initiated following the ingestion of contaminated drinking water containing the intermediate host of the parasite carrying guinea worm larva (Figure 1).
Water fleas living in water bodies such as ponds and other stagnant water feeds on the larva of D. medinensis. After drinking contaminated water containing copepods, the larva of guinea worm are immediately released from the copepods in the stomach of the infected individual. Rhabditiform larva starts making their way through the duodenal wall or digestive tract and into the body cavity of the host.
The invading female larva grows into adult stage (measuring about 2-3 feet long), and stimulate a blister (sore) and ulcer on the legs and feet. The sore ruptures within 2-3 days to release fluids and the female worm, and this is also accompanied by a painful sensation and other clinical episodes in the affected host (Figure 2).
It is noteworthy that the fluids (which are typically bacteriologically sterile) contain millions of immature larvae and white blood cells. Release of larva contaminates surrounding water bodies (i.e. when affected individuals comes in contact with water bodies), and this starts the cycle of D. medinensis again following the ingestion of larva by Cyclops.
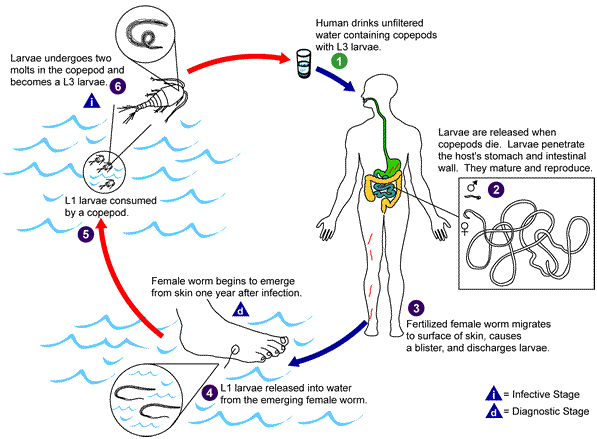
Infective stage of D. medinensis develops in the Cyclops and other intermediate hosts of the parasite. The crippling effects of GWD occur when the joints become affected (causing arthritis) and when secondary bacterial infections which can cause septicaemia and other bacterial related infections develop.
Laboratory diagnosis of dracunculiasis
Guinea worm disease is a localized infection that is peculiar to a given geographical region. The diagnosis of GWD is chiefly based on the clinical presentations associated with dracunculiasis, thus its identification in the laboratory is made following the rupturing of blisters and the release of female worms accompanied by fluids containing larva. Microscopic examination of the fluids reveal moving larva of the parasite. No serologic test is available for the diagnosis of GWD.
Treatment of dracunculiasis
There are currently no curative drugs of choice for the management of GWD. However, metronidazole and mebendazole are occasionally used in dracunculiasis endemic regions.
Surgical extraction of the worm before the formation of blisters or its mechanical removal from the leg or feet using a stick on which the protruding worm is coiled on and wind out a few centimeters every day until the whole worm is removed are typically the best management practice for handling GWD.
Mechanical worm extraction using sticks can last for several days or weeks. Analgesics (e.g. aspirin) and anti-bacterial agents can also be administered to take care of the pain and secondary bacterial infections of the disease respectively.
Control and prevention of dracunculiasis
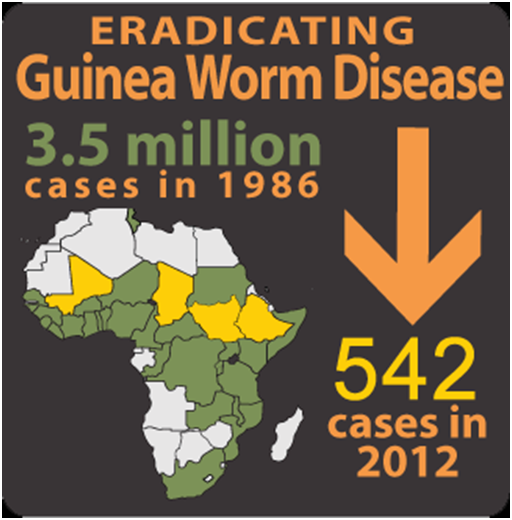
Currently, notable cases of dracunculiasis have significantly dropped in its rate of occurrence across the globe owing to the global efforts directed at eradicating the disease. In 1986, the cases of guinea worm disease (GWD) per year across the globe were put at about 3.5 million, but in 2012, the global cases of GWD known per year was 542 (Figure 3).
The eradication of GWD from the face of the earth is possible in the near future owing to the global efforts in containing the disease. GWD has been officially declared eliminated in some formerly endemic countries such as Pakistan, some African countries and India. Guinea worm pipe filters are used to drink water from the streams or ponds in guinea worm endemic areas (Figure 4).

Other countries in Africa such as Ghana and Nigeria, that were usually classified as GWD endemic countries have now been certified and declared free of the disease by the International Commission for the Certification of Dracunculiasis Eradication (ICCDE). ICCDE is team of GWD specialists established by WHO in 1995, and who are saddled with the sole responsibility of verifying and confirming the status of GWD endemic countries who are applying for certification to be considered guinea worm free.
Since 1986 when the global guinea worm eradication program (GWEP) began, the number of cases of GWD has been drastically reduced across the world, and many countries have been certified free from the disease while some are still under the pre-certification stage. However, the disease still remains endemic in only four African countries including South Sudan, Mali and Chad and Ethiopia. Cote d’Ivoire, Ghana, Kenya, Niger, Nigeria, and Sudan where dracunculiasis used to be endemic are currently in the pre-certification stage of GWD eradication.
Nevertheless, Nigeria was declared guinea-worm free in 2013 by the World Health Organization. The ICCDE harm of WHO certifies a country free from dracunculiasis after that country maintains adequate nationwide surveillance for at least 3 consecutive years and demonstrates that no cases of indigenous dracunculiasis occurred during that period. The spread of GWD in some sub Saharan African countries is controlled by case containment centers (Figure 5) which are localized and specialized to handle cases of dracunculiasis at the community level.
Local case containment centers are manned by seasoned local healthcare providers who provide assistance to GWD patients in the area of treatment and management of the infection so that they do not contaminate nearby water sources. In guinea worm endemic regions, filter pipes fitted with fine-mesh material are used to drink water from ponds or other water sources (Figure 6) in order to prevent the direct ingestion of copepods which contain the infective stage of the parasite.
People who live in dracunculiasis endemic regions and who drink from ponds and water sources contaminated by persons with GWD are at risk of acquiring the disease. GWD can be controlled and prevented by constantly drinking clean water, providing safe drinking water in endemic areas, boiling and filtering of potentially contaminated drinking water before use and educating residents in GWD endemic regions about the disease.


Contaminated water bodies should be treated with chemicals to destroy Cyclops, the intermediate host of the parasite. Persons with an emerging female worm of D. medinensis should be prevented or persuaded from entering drinking water ponds or other sources of drinking water in order to reduce the spread and transmission of the disease.
References
Aschengrau A and Seage G.R (2013). Essentials of Epidemiology in Public Health. Third edition. Jones and Bartleh Learning,
Beers M.H., Porter R.S., Jones T.V., Kaplan J.L and Berkwits M (2006). The Merck Manual of Diagnosis and Therapy. Eighteenth edition. Merck & Co., Inc, USA.
Chiodini P.L., Moody A.H., Manser D.W (2001). Atlas of medical helminthology and protozoology. 4th ed. Edinburgh: Churchill Livingstone.
Dictionary of Microbiology and Molecular Biology, 3rd Edition. Paul Singleton and Diana Sainsbury. 2006, John Wiley & Sons Ltd. Canada.
Ghosh S (2013). Paniker’s Textbook of Medical Parasitology. Seventh edition. Jaypee Brothers Medical Publishers,
Gillespie S.H and Pearson R.D (2001). Principles and Practice of Clinical Parasitology. John Wiley and Sons Ltd. West Sussex, England.
Gordis L (2013). Epidemiology. Fifth edition. Saunders Publishers, USA.
John D and Petri W.A Jr (2013). Markell and Voge’s Medical Parasitology. Ninth edition.
Kumar V, Abbas A.K, Fausto N and Aster A (2009). Robbins and Cotran Pathologic Basis of Disease. 8th edition. W.B. Saunders Co, USA.
Lee JW (2005). Public health is a social issue. Lancet. 365:1005-6.
Leventhal R and Cheadle R.F (2013). Medical Parasitology. Fifth edition. F.A. Davis Publishers,
Lucas A.O and Gilles H.M (2003). Short Textbook of Public Health Medicine for the tropics. Fourth edition. Hodder Arnold Publication, UK.
MacMahon B., Trichopoulos D (1996). Epidemiology Principles and Methods. 2nd ed. Boston, MA: Little, Brown and Company. USA.
Mandell G.L., Bennett J.E and Dolin R (2000). Principles and practice of infectious diseases, 5th edition. New York: Churchill Livingstone.
Molyneux, D.H., D.R. Hopkins, and N. Zagaria (2004) Disease eradication, elimination and control: the need for accurate and consistent usage. Trends Parasitol, 20(8):347-51.
Nelson K.E and Williams C (2013). Infectious Disease Epidemiology: Theory and Practice. Third edition. Jones and Bartleh Learning
Roberts L, Janovy J (Jr) and Nadler S (2012). Foundations of Parasitology. Ninth edition. McGraw-Hill Publishers, USA.
Schneider M.J (2011). Introduction to Public Health. Third edition. Jones and Bartlett Publishers, Sudbury, Massachusetts, USA.
Discover more from #1 Microbiology Resource Hub
Subscribe to get the latest posts to your email.


