Paragonimiasis or lung-fluke infection is a protozoan lung disease that resembles bacterial tuberculosis in humans; and it is caused by trematodes or flukes. It is a lung-fluke disease that has a worldwide distribution but more prevalent in parts of Asia including China, Taiwan, Indonesia and Japan.
The disease has also been reported in some parts of Africa such as Cameroon and Nigeria as well as in South and Central America. Paragonimiasis is an inflammatory pulmonary disease that is preventable and caused by certain species of flukes. Flukes are flatworms (i.e. platyhelminthes) that morphologically bear the shape of a leaf. There are a wide variety of flukes or flatworms that parasitize humans apart from those that cause paragonimiasis (e.g. Fasciola, Clonorchis, and Opisthorchis).
Human infection with the causative agent of paragonimiasis is usually initiated following the consumption of raw or undercooked crabs or shellfish containing the infective form of the parasite. Paragonimiasis is caused by Paragonimus species. The Paragonimus species that are known to parasitize humans include:
- P. westermani (the main causative agent of paragonimiasis in Asia)
- P. africanus
- P. heterotremus
- P. mexicanus
- P. uterobilateralis
Paragonimus species are trematodes of major importance unlike other flukes such as Fasciola which are of least medical importance to humans.
Type and morphology of Paragonimus species
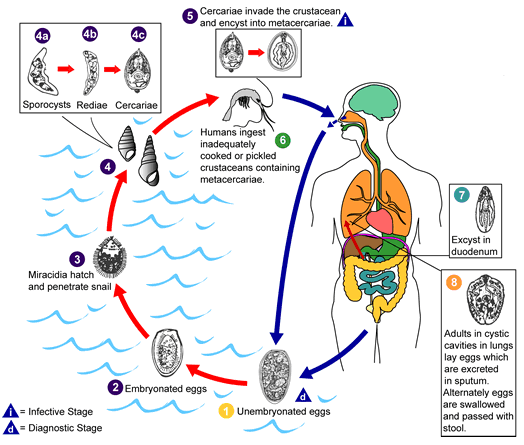
Paragonimus species exist in various morphological forms. The organism exist as metacercariae (the infective form) in its second intermediate host (e.g. crab); as miracidia (singular: miracidium) in its first intermediate host (i.e. snail); and as mature worms or flukes in its human host. Miracidia undergoes different morphological changes within the snail to form sporocysts, rediae and many cercariae.
Vector, reservoir and habitat of Paragonimus species
Paragonimus species has no insect vector. The parasite is naturally reserved in snails (as miracidia) and in crayfish, shellfish, crabs and other crustaceans as cercariae. Domestic and wild animals are reservoir hosts of Paragonimus species. Definitive hosts of Paragonimus species may include carnivorous animals such as cats, rats and dogs.
Snails are the first intermediate host of Paragonimus species while crayfish, freshwater crabs and other crustaceans are the second intermediate hosts of the parasite. Eggs of Paragonimus species swim freely in freshwater after being shed in the feaces or sputum of infected persons. Animals that feed on freshwater crustaceans could also serve as hosts of Paragonimus flukes; and these could serve as route via which human infection occurs.
Clinical signs and symptoms of Paragonimus species
Paragonimiasis is a pulmonary disease caused by protozoan species of the genus Paragonimus. Clinical signs and symptoms of paragonimiasis some of which may resemble bacterial-associated pulmonary diseases include chronic coughing up blood, chest pain, bloody diarrhea, dyspnea, sputum expectoration containing blood, recurrent bacterial pneumonia, night sweats and persistent coughing.
Other complications of paragonimiasis occurs when the parasite invade the CNS; and this could result to meningitis, oedema, haemorrhage of the cerebral cortex and severe headache. Vomiting, diarrhea and liver disease normally ensue when Paragonimus species invades the GIT especially the intestines and liver.
Some Paragonimus species may be retained in the walls of the intestines from where they migrate to other organs such as the kidney, liver, skeletal muscles and pancreas to cause extra-pulmonary infections. Bronchitis, a secondary infection usually ensues following the rupture of Paragonimus cysts in the lungs especially the bronchi. Chronic pulmonary abscess in paragonimiasis may resemble tuberculosis.
Pathogenesis of Paragonimus species infection
Paragonimus species is transmitted to humans after eating raw or undercooked crabs, crayfish or shellfish and other infected crustaceans (Figure 1). Human infections with the parasite can be asymptomatic or symptomatic depending on the magnitude of Paragonimus invasion. Paragonimiasis is endemic in places where crayfish, crabs and shellfish are ingested as a delicacy either raw or in juicy forms.
After ingestion, metacercariae of Paragonimus species in raw or undercooked crayfish or crab excyst in the duodenum or small intestine, and the parasite burrows through the walls of the gut into the peritoneal cavity from where they reach the lungs. In the lungs, the metacercariae develops into mature flukes as paired worms in cyst i.e. they become encapsulated in pairs. The worms exist in a fibrous capsule that communicates with the bronchioles in the lungs.
There is usually expectoration of bloody sputum due to secondary bacterial infection which is common with the disease. Other sites of the body such as the brain, liver and spleen may be infected when the flukes leave the lungs. Eggs of Paragonimus species which are later excreted in the sputum or feaces of infected individuals are produced few months after infection.
On reaching freshwater (especially via feacal contamination of water habitat) the eggs of Paragonimus hatch to release miracidia which swims in the freshwater to locate a suitable snail host (Figure 1). Miracidia or miracidium undergoes series of biological changes in the snail to release cercariae which encyst in crayfish or crabs to form metacercariae. In this manner, the life cycle of Paragonimus species is perpetuated in a defined human population; and paragonimiasis can last for several years in humans especially if not treated well.
Laboratory diagnosis of Paragonimus species
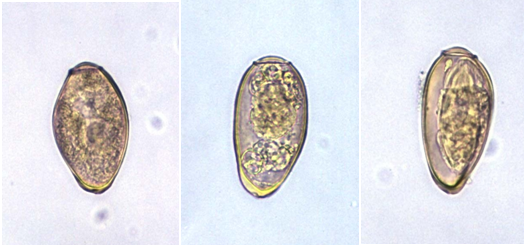
The laboratory diagnosis of paragonimiasis is usually based on identification of the eggs of Paragonimus species (Figure 2) in feaces, sputum and other clinically important specimen using microscopy and concentration techniques. Antigen detection techniques and serodiagnosis are also available for the laboratory diagnosis of paragonimiasis since eggs of Paragonimus species are usually absent and difficult to find in sputum and other clinical specimens few months after infection.
Treatment, control and prevention of Paragonimus species infection
Paragonimiasis can be well treated using antiprotozoal agents such as praziquantel and bithionol. Praziquantel and bithionol are usually the drugs of choice for treating the infection. Paragonimiasis is usually a parasitic disease of the Asian continent especially the Far Eastern part of Asia such as Japan, Philippines, Korea, Indonesia and China amongst others but the disease is now occasionally being reported in some parts of Africa and South America with low occurrence.
Infection is highest in endemic areas especially in regions where children play and ingest raw or undercooked crabs, shellfish and other crustaceans. Paragonimiasis as earlier said is a preventable parasitic disease. People should take necessary precautions to avoid infection with the protozoan. Raw or undercooked crayfish, crabs, shellfish or other infected crustaceans should not be eaten unless when properly cooked as they may contain the infective form of the parasite (i.e. metacercariae).
Infected persons should be properly treated; and defeacation in rivers and other water sources should be discouraged by providing good toilets and latrines especially in public places. Advocacy and education of the general public is critical to the prevention of the disease especially in endemic regions. Adequate cooking of crayfish, crabs, shellfish and other crustaceans before consumption is critical to preventing infection with Paragonimus species.
References
Aschengrau A and Seage G.R (2013). Essentials of Epidemiology in Public Health. Third edition. Jones and Bartleh Learning,
Beers M.H., Porter R.S., Jones T.V., Kaplan J.L and Berkwits M (2006). The Merck Manual of Diagnosis and Therapy. Eighteenth edition. Merck & Co., Inc, USA.
Chiodini P.L., Moody A.H., Manser D.W (2001). Atlas of medical helminthology and protozoology. 4th ed. Edinburgh: Churchill Livingstone.
Dictionary of Microbiology and Molecular Biology, 3rd Edition. Paul Singleton and Diana Sainsbury. 2006, John Wiley & Sons Ltd. Canada.
Ghosh S (2013). Paniker’s Textbook of Medical Parasitology. Seventh edition. Jaypee Brothers Medical Publishers,
Gillespie S.H and Pearson R.D (2001). Principles and Practice of Clinical Parasitology. John Wiley and Sons Ltd. West Sussex, England.
Gordis L (2013). Epidemiology. Fifth edition. Saunders Publishers, USA.
John D and Petri W.A Jr (2013). Markell and Voge’s Medical Parasitology. Ninth edition.
Kumar V, Abbas A.K, Fausto N and Aster A (2009). Robbins and Cotran Pathologic Basis of Disease. 8th edition. W.B. Saunders Co, USA.
Lee JW (2005). Public health is a social issue. Lancet. 365:1005-6.
Leventhal R and Cheadle R.F (2013). Medical Parasitology. Fifth edition. F.A. Davis Publishers,
Lucas A.O and Gilles H.M (2003). Short Textbook of Public Health Medicine for the tropics. Fourth edition. Hodder Arnold Publication, UK.
MacMahon B., Trichopoulos D (1996). Epidemiology Principles and Methods. 2nd ed. Boston, MA: Little, Brown and Company. USA.
Mandell G.L., Bennett J.E and Dolin R (2000). Principles and practice of infectious diseases, 5th edition. New York: Churchill Livingstone.
Molyneux, D.H., D.R. Hopkins, and N. Zagaria (2004) Disease eradication, elimination and control: the need for accurate and consistent usage. Trends Parasitol, 20(8):347-51.
Nelson K.E and Williams C (2013). Infectious Disease Epidemiology: Theory and Practice. Third edition. Jones and Bartleh Learning
Roberts L, Janovy J (Jr) and Nadler S (2012). Foundations of Parasitology. Ninth edition. McGraw-Hill Publishers, USA.
Schneider M.J (2011). Introduction to Public Health. Third edition. Jones and Bartlett Publishers, Sudbury, Massachusetts, USA.
Discover more from #1 Microbiology Resource Hub
Subscribe to get the latest posts to your email.


