Tetracyclines are general purpose antibiotics used for a variety of clinical applications, and they include tetracycline, chlortetracycline, oxytetracycline, dimethyl chlortetracycline, minocycline and doxycycline. Antibiotics in this category are protein synthesis inhibitors like the macrolides, but tetracyclines bind to the 30S ribosomal subunit of bacterial ribosomes during protein synthesis. They are used to treat a wide variety of bacterial-related infections.
SOURCES OF TETRACYCLINES
The tetracyclines are naturally synthesized from the bacteria genus Streptomyces. The species of Streptomyces that synthesize tetracyclines naturally include S. aureofaciens and S. rimosus which synthesize chlortetracycline and oxytetracycline or dimethyl chlortetracycline respectively. Tetracyclines can also be synthesized by semi-synthetic and other synthetic processes.
STRUCTURE OF TETRACYCLINES
The general structure of the tetracyclines is composed of a four-fused benzene ring (Figure 1) to which substituent groups are added to form newer tetracyclines such as minocycline and doxycycline. The 4-fused benzene ring is the basis for the synthesis of newer tetracyclines through semi-synthetic or synthetic processes.
CLINICAL APPLICATION OF TETRACYCLINES
Tetracyclines are usually used for blind treatment in clinical medicine i.e. a type of treatment administered prior to formal laboratory test results. They are often the drug of choice for some bacterial infections such as those caused by Brucella, Chlamydia, Vibrio, Francisella and Yersinia amongst others.
SPECTRUM OF ACTIVITY OF TETRACYCLINES
The tetracyclines are broad spectrum antibiotics, and they have antibacterial activity against pathogenic Gram positive bacteria, Gram negative bacteria and other aerobic and anaerobic bacteria. They are bacteriostatic in action.
MECHANISM OR MODE OF ACTION OF TETRACYCLINES
Tetracyclines are generally protein synthesis inhibitors, and they act by preventing the binding of aminoacyl tRNA to the acceptor (A) site on bacterial ribosome. The binding of tetracyclines to the 30S ribosomal subunit blocks the attachment of aminoacyl-tRNA to the acceptor site on mRNA (i.e. the 30S ribosomal subunit), and this interferes with protein synthesis by inhibiting the elongation of the polypeptide chain. The antibiotic is very effective on susceptible pathogenic bacteria than those that are resistant to it. Tetracyclines are active against a wide variety of pathogenic bacteria as well as intracellular parasites such as Chlamydia and Rickettsia and some protozoa.
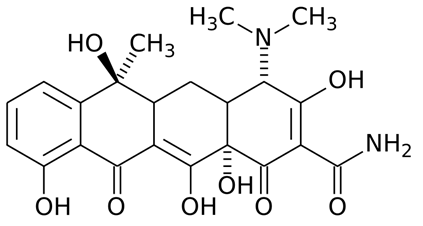
Figure 1. General structure of tetracyclines. The 4-fused benzene ring of the tetracyclines (also known as naphthalene) is unique because it contains 3 side chains or R-sites to which substituent groups are added to form new tetracyclines (e.g. doxycycline).
BACTERIAL RESISTANCE TO TETRACYCLINES
The antibacterial activity of the tetracyclines is usually dependent on the intracellular concentration of the drug by susceptible pathogenic bacteria. However, pathogenic bacteria that fail to concentrate the antibiotic intracellularly become resistant to the agent. The alteration of the cell membrane of the target bacteria (usually by the presence of resistant plasmid) and inactivation of the antibiotic by bacterial enzymes can compromise the clinical efficacy of the tetracyclines. The later tetracyclines such as doxycycline and minocycline are used to counter the problem of resistance associated with the earlier tetracyclines (e.g. chlortetracycline).
PHARMACOKINETICS OF TETRACYCLINES
Tetracyclines are readily absorbed from the GIT when orally administered and they are well distributed in the tissues of the body. But the antibiotic penetrates the CSF poorly, and has little or no clinical efficacy against meningococcal infections. Tetracyclines are excreted in their inactive forms in urine, bile and feaces. The tetracyclines are chelated by some foods especially dairy products (e.g. milk) that contain divalent cations; and thus they should not be co-administered with such foods. The absorption of tetracyclines by the body and their antibacterial activity can also be compromised when co-administered with antacid or other chemical substances that treat food indigestion.
SIDE EFFECT/TOXICITY OF TETRACYCLINES
The untoward effect associated with the clinical usage of tetracyclines is minimal, and the antibiotic has a low toxicity compared to other antibacterial agents. However, the use of tetracyclines in children (between ages 0-8 years old) has been associated with tooth decolouration (seen as yellowing of the tooth) and impairment of bone development due to the high affinity of the antibiotic for calcium, the main component of bones and teeth.
Tetracycline is also contra-indicated for pregnant women because the antibiotic reacts with calcium phosphate when administered for a long time; and this generally affects bone development in the foetus. Other side effects associated with the use of tetracyclines include nausea, vomiting, skin rashes, diarrhea, and disruption of the normal bacterial flora in the intestines. Alteration of the normal flora of the intestines by the overuse or prolonged usage of tetracyclines predisposes the recipient human hosts to other bacterial-related infections and opportunistic diseases (e.g. candidiasis).
References
Ashutosh Kar (2008). Pharmaceutical Microbiology, 1st edition. New Age International Publishers: New Delhi, India.
Axelsen P. H (2002). Essentials of Antimicrobial Pharmacology. Humana Press, Totowa, NJ.
Balfour H. H (1999). Antiviral drugs. N Engl J Med, 340, 1255–1268.
Bean B (1992). Antiviral therapy: current concepts and practices. Clin Microbiol Rev, 5, 146–182.
Beck R.W (2000). A chronology of microbiology in historical context. Washington, D.C.: ASM Press.
Champoux J.J, Neidhardt F.C, Drew W.L and Plorde J.J (2004). Sherris Medical Microbiology: An Introduction to Infectious Diseases. 4th edition. McGraw Hill Companies Inc, USA.
Chemotherapy of microbial diseases. In: Chabner B.A, Brunton L.L, Knollman B.C, eds. Goodman and Gilman’s The Pharmacological Basis of Therapeutics. 12th ed. New York, McGraw-Hill; 2011.
Chung K.T, Stevens Jr., S.E and Ferris D.H (1995). A chronology of events and pioneers of microbiology. SIM News, 45(1):3–13.
Courvalin P, Leclercq R and Rice L.B (2010). Antibiogram. ESKA Publishing, ASM Press, Canada.
Denyer S.P., Hodges N.A and Gorman S.P (2004). Hugo & Russell’s Pharmaceutical Microbiology. 7th ed. Blackwell Publishing Company, USA. Pp.152-172.
Dictionary of Microbiology and Molecular Biology, 3rd Edition. Paul Singleton and Diana Sainsbury. 2006, John Wiley & Sons Ltd. Canada.
Drusano G.L (2007). Pharmacokinetics and pharmacodynamics of antimicrobials. Clin Infect Dis, 45(suppl):89–95.
Engleberg N.C, DiRita V and Dermody T.S (2007). Schaechter’s Mechanisms of Microbial Disease. 4th ed. Lippincott Williams & Wilkins, Philadelphia, USA.
Finch R.G, Greenwood D, Norrby R and Whitley R (2002). Antibiotic and chemotherapy, 8th edition. Churchill Livingstone, London and Edinburg.
Ghannoum MA, Rice LB (1999). Antifungal agents: Mode of action, mechanisms of resistance, and correlation of these mechanisms with bacterial resistance. Clin Microbiol Rev, 12:501–517.
Gillespie S.H and Bamford K.B (2012). Medical Microbiology and Infection at a glance. 4th edition. Wiley-Blackwell Publishers, UK.
Gordon Y. J, Romanowski E.G and McDermitt A M (2005). A review of antimicrobial peptides and their therapeutic potential as anti-infective drugs. Current Eye Research, 30(7): 505-515.
Hardman JG, Limbird LE, eds. Goodman and Gilman’s The Pharmacological Basis of Therapeutics. 10th ed. New York: McGraw-Hill; 2001.
Joslyn, L. J. (2000). Sterilization by Heat. In S. S. Block (Ed.), Disinfection, Sterilization, and Preservation (5th ed., pp. 695-728). Philadelphia, USA: Lippincott Williams and Wilkins.
Katzung, B. G. (2003). Basic and Clinical Pharmacology (9th ed.). NY, US, Lange.
Kontoyiannis D.P and Lewis R.E (2002). Antifungal drug resistance of pathogenic fungi. Lancet. 359:1135–1144.
Discover more from #1 Microbiology Resource Hub
Subscribe to get the latest posts to your email.


