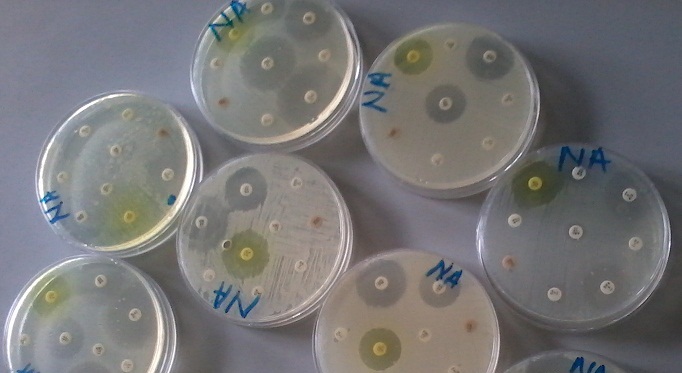
Quality control strains (or reference strains)are typed cultures of microorganisms with known antimicrobial susceptibility patterns and which are used to ensure consistency, accuracy and reproducibility of a particular susceptibility test. They are important in any disk diffusion test because susceptibility tests are affected by plethora of factors such as incubation temperature, size of the test organism and test medium.
When antimicrobial susceptibility is to be carried out, it is imperative that the clinical microbiologist also run a test using the reference strain corresponding to the test culture. Quality control strains helps to balance the result of an antibiogram. And if the result obtained for a given control strain against a particular antibiotic is outside the specified range (as given by the manufacturer or CLSI/NCCLS guidelines), the patient’s susceptibility test result should not be reported and the disk/antibiotic should not be used until the reason for the discrepancy is deciphered.
Reasons for poor performance of a control strain may include media used, inoculum size, incubation temperature and incorrect storage of disks or control strain. Quality control strains are commercially available as pellets of desiccated pure cultures, and are made into suspensions prior to their usage by following the manufacturer’s instruction. Reference strains of microorganisms can be obtained from national culture collection centers e.g. American Type Culture Collection (ATCC).
Examples of some quality control organisms include:
- Escherichia coli ATCC 25922
- Klebsiella pneumoniae ATCC 700603
- Pseudomonas aeruginosa ATCC 27853
- Staphylococcus aureus ATCC 25923
- Candida albicans ATCC 10231
- Haemophilus influenzae ATCC 33930
- Streptococcus pneumoniae ATCC 27336
- Enterococcus faecalis ATCC 29212 and so on.
References
Doern G, Vautour R, Gaudet M, Levy B (1994). Clinical impact of rapid in vitro susceptibility testing and bacterial identification. J Clin Microbiol, 32:1757–1762.
Doern G.V (1995). Susceptibility tests of fastidious bacteria. Manual of Clinical Microbiology, 6th edition, Murray P.R, Baron E.J, Pfaller M.A, Tenover F.C, Yolken R, American Society for Microbiology, Washington DC, Pp. 1342-1349.
Madigan M.T., Martinko J.M., Dunlap P.V and Clark D.P (2009). Brock Biology of Microorganisms, 12th edition. Pearson Benjamin Cummings Inc, USA.
Mahon C. R, Lehman D.C and Manuselis G (2011). Textbook of Diagnostic Microbiology. Fourth edition. Saunders Publishers, USA.
National Committee for Clinical Laboratory Standards. Performance Standards for antimicrobial susceptibility testing. 8th Informational Supplement. M100 S12. National Committee for Clinical Laboratory Standards, 2002. Villanova, Pa.
Washington J.A (1993). Rapid antimicrobial susceptibility testing: technical and clinical considerations. Clin Microbiol Newsl, 15:153–155.
Discover more from #1 Microbiology Resource Hub
Subscribe to get the latest posts to your email.