Leishmaniasis is the parasitic disease caused by the protozoal organism, Leishmania. The disease affects the skin, spleen and the liver, and it is usually characterized by extensive lesions on the skin (in the mouth, throat and nose region) that sometimes leads to deformity of the affected body part. Leishmaniasis can be old world or new world disease depending on the Leishmania species responsible for the disease and the part of the world where the disease occurred. Old world leishmaniasis is caused by Leishmania species including L. donovani, L. infantum, L. major, L. tropica, and L. aethiopica; and these parasites occur mainly in Asia, Europe and Africa (which are referred to as old world).
New world leishmaniasis is caused by Leishmania species including L. braziliensis, L. mexicana, L. guyanensis, L. amazonensis, L. panamensis and L. peruviana; and they occur only in the Americas (south and central), which are referred to as the new world.New world leishmaniasis is transmitted by species of sand flies in the genus Lutzomyia while old world leishmaniasis is transmitted by sand fly species in the genus Phlebotomus. Leishmania species are parasitic organisms that reside within the phagocytes of humans or animals as intracellular amastigotes. But in the insect vector (sand fly) that transmits the parasite, the organism lives as extracellular promastigotes in the midgut or stomach of the sand fly. Leishmaniasis is usually presented clinical in any of the following three (3) forms:
- Visceral Leishmaniasis (kala-azar):- It is caused by Leishmania donovani and other related Leishmania species. Kala-azar (which is a systemic type of leishmaniasis) is a more severe type of leismaniasis which mainly affects the reticulo-endothelial system (RES) of the body resulting in massive splenomegaly and hepatomegaly.
- Cutaneous Leishmaniasis (oriental sore):- Cutaneous leishmaniasis is caused by Leishmania species including L. tropica and L. guyanensis. It can also be called Baghdad sore, dry cutaneous ulcer, wet cutaneous sore and so on depending on the place and type of lesion produced. Cutaneous leishmaniasis is usually characterized by ulceration and necrosis on the overlying skin, and it is a localized type of leishmaniasis.
- Mucocutaneous Leishmaniasis:- This type of leishmaniasis usually involves the oral and nasopharyngeal mucosa of the body. Mucocutaneous leishmaniasis, a localized type of infection can occur following a primary skin infection leishmaniasis. The Leishmania species implicated include L. braziliensis. Leishmaniasis is a parasitic disease that is caused by parasites in the genus: Leishmania. The different species of Leishmania that cause leishmaniasis include:
- Leishmania donovani
- L. tropica
- L. major
- L. Mexicana
- L. braziliensis
- L. guyanensis
Type and morphology of Leishmania
Leishmania species are hemoflagellated protozoal parasites. The term hemoflagellate is used to describe blood-borne parasites i.e. protozoal organisms that invade and live in the blood of their hosts. Such parasites can also be found in the tissues (e.g. tissues of bone marrow, liver and spleen) of their hosts. Leishmania species also have a whip-like structure called flagella which they use for locomotion. Morphologically, Leishmania species have two distinct morphological forms: the amastigotes and the promastigotes. The amastigotes are nonmotile (nonflagellated) forms of Leishmania species that occurs only in humans and other mammals.
Amastigotes (which are normally intracellular in the host) are 3-6 µm long and 1.5-3 µm in diameter, and they are the only forms of Leishmania species that infect man and other animals. The promastigotes are the infective forms of Leishmania species that are transmitted or introduced into the skin of humans by the insect vector of the parasites. Promastigotes are flagellated forms of Leishmania species, and they are motile and occur only in the insect vector (sand fly) that transmits the parasite to humans and other mammals.
Vector, reservoir and habitat of Leishmania
The natural agent that helps in transmitting Leishmania species from animals to humans or between humans is called sand fly. Sand flies are the natural insect vectors of Leishmania parasites. This class of insects is relatively weak insects, and they only fly by making short jumps. Leishmania parasites are transmitted to humans through the bite of an infected sand fly. The insect vector (sand fly) lives in excreta, burrows of rodents, rotting leaves of trees and in termite hills.
Sand flies feed on plant nectars but the female sand flies require blood meal for their egg development, and thus suck the blood of humans and other mammals to meet this developmental demand. Their feeding time is usually in the night or in the beginning of the day. During blood meal, the Leishmania parasite is introduced and inoculated into the skin of humans, from which they go on to affect other tissues of the body including the skin.
Phlebotomus and Lutzomyia are the two genera of sand flies which Leishmania parasites use as their intermediate hosts. Rodents (rat) and canines (dog) are the primary reservoirs of Leishmania species. Sand flies in the genera Phlebotomus transmit Leishmania parasites in only Asia, Europe and Africa while those in the Lutzomyia genera (L. longipalpis) only transmit Leishmania parasites in the Americas (central and south). Upon blood meal, the sand fly picks up Leishmania parasites (amastigotes) from an infected person or animal.
The ingested amastigotes are transformed into promastigotes in the stomach or midgut of the insect vector where they multiply actively until further transmission to a susceptible human or animal host. Some Leishmania infections are zoonotic in nature (those caused by L. panamensis and so on). They use animals such as dogs, foxes and forest rodents as their reservoirs and these can serve as route via which the disease can be transmitted to humans. Humans can also serve as reservoirs for Leishmania parasites, and they usually become infected by the parasite after visiting an area that is known to be endemic with the disease.
Clinical signs and symptoms of Leishmania
The clinical expression of leishmania is usually dependent on the immune system response of the affected patient and the virulence and pathogenicity of the Leishmania parasite. Clinically, the signs and symptoms of leishmaniasis may include irregular and chaotic fever, growing weakness, continuous emaciation, spontaneous and progressive skin and mucosa lesions, splenomegaly (swelling of the spleen), hepatomegaly (swelling of the liver), diarrhea in some cases, anaemia, epistaxis (nose bleeding), kidney damage and gum bleeding. The signs and symptoms of the disease though similar across the different clinical forms of leishmaniasis (kala-azar, cutaneous and mucocutaneous leishmaniasis) to some extent; the different clinical forms of leishmaniasis as elaborated above usually have their unique signs and symptoms that are peculiar to them.
Pathogenesis of Leishmania infection
The clinical episode of leishmaniasis begins following the transmission or inoculation of the Leishmania parasite into an individual’s body after the bite of a female sand fly (insect vector for Leishmania parasites). The infected female sand fly transmits the infective promastigotes into the host’s body through a bite usually during a blood meal (Figure 1). The motile promastigote becomes phagocytosed by macrophages, and then changes into nonmotile forms known as amastigotes. The amastigotes (which are intracellular forms of the parasite) multiply within the macrophages, ruptures and releases Leishmania parasites that go on to infect new macrophages. They are again phagocytosed and the life cycle of the Leishmania parasite continues in this manner. Leishmaniasis usually has three clinical forms viz: visceral leishmaniasis (kala-azar), cutaneous leishmaniasis (oriental sores) and mucocutaneous leishmaniasis (espundia).
In kala-azar, the amastigotes multiply in the macrophages of the liver, bone marrow, spleen, the lymph nodes and other tissues of the reticulo-endothelial system (RES). Kala-azar is a systemic type of leishmaniasis i.e. the parasite spreads from its site or point of inoculation to other vital tissues of the body including the blood; and it affects mainly the RES tissues. It is also worth mentioning that kala-azar is an Indian local name which actually means black sickness, and which was used as a suggestion or reference name that was given to the disease following the discovery that patients suffering from leishmaniasis do produce grayish colour secretions from their skin. Kala-azar is caused by specific Leishmania species including L. donovani and L. infantum (that is also called L. chagasi). Visceral leishmaniasis (kala-azar) infections are usually fatal and can result to death if left untreated. But some forms of the disease known as post kala-azar dermal leishmaniasis (PKDL) occurs in patients after some months or years of treatment and recovery from the disease (kala-azar).
In PKDL, there is obvious reappearance of raised erythematous lesions (containing abundant Leishmania parasites) on the skin of the affected patient. PKDL is region specific, and occurs in only parts of East Africa and Asia (India). Cutaneous leishmaniasis unlike kala-azar is a localized type of leishmaniasis. The disease is specifically limited to a small area of the skin in the affected patient’s body, and this result to several lesions on different parts of the body. Cutaneous leishmaniasis which is usually chronic and self-limiting occurs in about 2 weeks following the bite of an infected female sand fly. A small cutaneous lesion (known as papule) which later progress into an ulcerative lesion appears on the skin.
The clinical presentations of cutaneous leishmaniasis are usually dependent and characterized by the species of parasite response of the patient and the region of the world where it occurs. For example, L. mexicana occur in Mexico and it causes chicleros ulcer, L. tropica occur in the dry region of the Eastern Hemisphere and it causes dry urban oriental sore, L. major and L. aethiopica causes oriental sores and so on. Mucocutaneous leishmaniasis which can also be called espundia or nasopharyngeal leishmaniasis affects mainly the mucosal parts of the body (e.g. the nose and the nasopharynx). The disease usually results in sow but progressive and far-reaching lesions along the mucosal area of the affected body part. L. braziliensis, L. guyanensis and L. panamensis are amongst the Leishmania species that cause espundia.
Espundia is usually initiated after the healing of cutaneous leishmaniasis, and the spreading of it to other parts of the body (especially the facial region of the body). Following the curing of cutaneous leishmaniasis, there appears some mucosal lesions (in some cases) years later on the nose and other mucosal parts of the affected patient. This can lead to the disfigurement of the nose and other affected nasopharyngeal organs (the larynx and pharynx) if not treated, and death can occur in some cases. It is noteworthy that recovery from some Leishmania infections (kala-azar) usually develops a permanent immunity in the individual.

Laboratory diagnosis of Leishmania infection
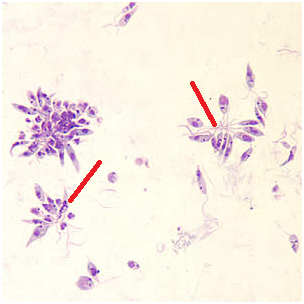
The laboratory and clinical recognition of leishmaniasis is usually deciphered by some specific signs and symptoms of the disease coupled with a history of the affected individual in areas where the infection is widespread. Leishmaniasis is diagnosed in the laboratory by microscopical investigations, culture techniques, identification of the parasite’s amastigotes (Figure 2) in tissue samples, and by serological investigations. The promastigotes of Leishmania spp. can also be identified from cultures under the microscope (Figure 3). The specimens used for the laboratory diagnosis of leishmaniasis include skin scrapings, tissue biopsies, lymph node aspirates, blond and puncture of the liver, bone marrow or spleen.

In microscopical investigations, the amastigotes of the parasites are sought after using Giemsa staining technique. Culture techniques can also be used when the parasite cannot be seen in smears. Special type of culture media (e.g. Novy, Nicolle MacNeal’s, NNN medium) in which are incorporated living cells or special nutritional supplements for the growth of the parasite. Serological techniques including ELISA and even PCR are combined in some cases to detect Leishmania parasites.
Treatment of Leishmania infection
The clinical symptoms of the disease and the species of Leishmania implicated are vital to recommendation of appropriate treatment measure for the leishmaniasis. Most drugs used for the treatment of leishmaniasis are drugs that contain pentavalent antimony, but most recently, liposomal-encapsulated drugs have been used in the treatment of some cases of leishmaniasis. Liposome-encapsulated drugs are effective in the treatment of leishmaniasis because the drug targets the macrophages, which are the site of the parasites amastigotes. The use of recombinant interferon-gamma (IFNλ) for the treatment of leishmaniasis is also applicable in some parts of the world. In such treatments, IFNλ is not used alone but in combination with pentavalent antimony (pentamidine).
The IFNs helps in recruiting and activating macrophages that destroys the intracellular forms of the parasite (amastigotes) in the affected patients. Amphotericin B (an antifungal agent) is also employed in the treatment of leishmaniasis. The reason for incorporating amphotericin B in leishmaniasis therapy is because the antifungal agent helps to destroy fungal organisms following any secondary infection in leishmaniasis. In addition, amphotericin B is believed to target and destroy the outer-membrane of the parasite (which is very rich in sterols). Ketoconazole and imidazole are other antifungal agents employed in leishmaniasis therapy. Topical antimicrobial agents are also used to treat some cutaneous and mucocutaneous forms of leishmaniasis.
Control and prevention of Leishmania infection
Leishmaniasis cannot thrive and spread in any population without the availability of the insect vector (infected sand flies of the Lutzomyia and Phlebotomus species), a reservoir of the parasite and most importantly a susceptible human host which usually provides the blood required for egg development in the insect vector. For the successful control and prevention of leishmaniasis and its possible eradication, it is very important that the life cycle of the parasite is well understood. In addition, the response of susceptible humans to the parasite and the mechanism of spread and distribution of the disease, the insect vector and its reservoirs must also be considered for the proper containment of the disease.
Since the parasite is primarily transmitted via the bite of an infected sand fly, controlling and reducing the number of the insect vector will help to contain the disease especially in endemic populations. Breeding sites of the insect vectors should be destroyed spraying insecticides and other chemicals that are capable of limiting their proliferation. People travelling to endemic areas should endeavour to wear proper clothing’s to cover their body in order to avoid bites from the insect vector of the parasite. Though there have been several attempts in the past to develop a vaccine for the disease, effective vaccine for the control and prevention of leishmaniasis is yet to be discovered.
References
Aschengrau A and Seage G.R (2013). Essentials of Epidemiology in Public Health. Third edition. Jones and Bartleh Learning,
Beers M.H., Porter R.S., Jones T.V., Kaplan J.L and Berkwits M (2006). The Merck Manual of Diagnosis and Therapy. Eighteenth edition. Merck & Co., Inc, USA.
Chiodini P.L., Moody A.H., Manser D.W (2001). Atlas of medical helminthology and protozoology. 4th ed. Edinburgh: Churchill Livingstone.
Dictionary of Microbiology and Molecular Biology, 3rd Edition. Paul Singleton and Diana Sainsbury. 2006, John Wiley & Sons Ltd. Canada.
Ghosh S (2013). Paniker’s Textbook of Medical Parasitology. Seventh edition. Jaypee Brothers Medical Publishers,
Gillespie S.H and Pearson R.D (2001). Principles and Practice of Clinical Parasitology. John Wiley and Sons Ltd. West Sussex, England.
Gordis L (2013). Epidemiology. Fifth edition. Saunders Publishers, USA.
John D and Petri W.A Jr (2013). Markell and Voge’s Medical Parasitology. Ninth edition.
Kumar V, Abbas A.K, Fausto N and Aster A (2009). Robbins and Cotran Pathologic Basis of Disease. 8th edition. W.B. Saunders Co, USA.
Lee JW (2005). Public health is a social issue. Lancet. 365:1005-6.
Leventhal R and Cheadle R.F (2013). Medical Parasitology. Fifth edition. F.A. Davis Publishers,
Lucas A.O and Gilles H.M (2003). Short Textbook of Public Health Medicine for the tropics. Fourth edition. Hodder Arnold Publication, UK.
MacMahon B., Trichopoulos D (1996). Epidemiology Principles and Methods. 2nd ed. Boston, MA: Little, Brown and Company. USA.
Mandell G.L., Bennett J.E and Dolin R (2000). Principles and practice of infectious diseases, 5th edition. New York: Churchill Livingstone.
Molyneux, D.H., D.R. Hopkins, and N. Zagaria (2004) Disease eradication, elimination and control: the need for accurate and consistent usage. Trends Parasitol, 20(8):347-51.
Nelson K.E and Williams C (2013). Infectious Disease Epidemiology: Theory and Practice. Third edition. Jones and Bartleh Learning
Roberts L, Janovy J (Jr) and Nadler S (2012). Foundations of Parasitology. Ninth edition. McGraw-Hill Publishers, USA.
Schneider M.J (2011). Introduction to Public Health. Third edition. Jones and Bartlett Publishers, Sudbury, Massachusetts, USA.
Discover more from #1 Microbiology Resource Hub
Subscribe to get the latest posts to your email.


