Isoniazid or isonicotinyl hydrazine (INH) is a first-line antibiotic used for the treatment of tuberculosis (TB), a droplet bacterial infection caused by Mycobacterium tuberculosis. TB is a highly infectious diseases that is mainly spread via the respiratory tract of infected persons to susceptible human hosts through sneezes, cough and saliva or aerosols that contain the infectious particle of the pathogenic organism. Other first-line anti-tuberculosis drugs include ethambutol, streptomycin, pyrazinamide and rifampicin.
The second-line drugs used for the treatment of tuberculosis especially in cases of drug resistance or when antimicrobial synergistic effect is warranted include para-aminosalicylic acid (PAS), cycloserine, kanamycin (an aminoglycoside), ethionamide and fluoroquinolones (e.g. ciprofloxacin). Isoniazid and other first-line anti-TB drugs are clinically effective because they act on the mycolic acid elements of the mycobacterial cell wall which is different from the cell wall of other pathogenic bacteria.
Isoniazid is a cell wall synthesis inhibitor, but it is only effective on the cell wall of Mycobacterium species (particularly those that cause TB) which contain a different type of element in their cell wall (i.e. mycolic acid). The presence of high content of mycolic acid exterior to the mycobacterial peptidoglycan layer makes the mycobacteria to be resistant to most antibacterial drugs inclusive of some anti-TB agents.
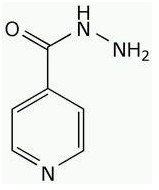
SOURCE AND STRUCTURE OF ISONIAZID
INH and other first-line anti-TB drugs are chemically synthesized antibacterial drugs. Anti-TB drugs are also produced by semi-synthetic processes. But rifampin is a semi-synthetic derivative of rifamycin, an antibiotic produced by Streptomyces mediterranei. The structure of isoniazid (INH) is a benzene nucleus (Figure 1).
SPECTRUM OF ACTIVITY OF ISONIAZID
Isoniazid and other anti-TB drugs are bactericidal inaction, and they interfere with the activities of the cell wall constituents of tubercle bacilli (especially their mycolic acid content).
CLINICAL APPLICATION OF ISONIAZID
Isoniazid (INH), rifampin (RIF), pyrazinamide (PZA) and ethambutol (EMB) or streptomycin are the main antibacterial agents used for the clinical management of tuberculosis (TB); and these drugs are used in combination with each other for the effective treatment of TB. Multiple drugs (inclusive of anti-TB drugs and other antibacterial agents) are usually used for the clinical management of tuberculosis because the administration of a single anti-TB drug often leads to the development of a bacterial population that will be resistant to that drug particular drug. And this is why most TB treatment today involves the administration of two or more effective drugs used simultaneously, and this helps to prevent the emergence of tubercle bacilli resistant to each of the drugs.
However, multidrug resistant tuberculosis (MDR TB) and extensively drug resistant TB (XDR TB) now exist, and these pathogens complicate the effective treatment of TB because they are virtually resistant to some of the known available drugs used for the clinical management of TB infections. The standard TB treatment according to WHO is normally administered in two phases viz: an initial phase which last for about 2-3 months using a combination of 3 anti-TB agents from the first-line TB drugs (inclusive of INH, RIF and EMB) and a continuation phase which spans a period about 8 months, and in which two of any of the first-line drugs are co-administered to the patient.
Both the initial stage and continuation stage are administered under a procedure known as directly observed therapy (DOT), a therapeutic procedure in which TB patients are given drugs based on the supervision of a health personnel or physician in a health center. DOT ensures that the TB patients follow the actual course of their therapy, as the possibility of not adhering strictly to it may arise due to the large number of drugs involved in TB treatment. Drugs are usually combined for TB treatment in order to achieve a synergistic effect, and such drug combination will help to reduce the development of resistance in the mycobacteria to any of the agent when used alone.
MECHANISM OR MODE OF ACTION OF ISONIAZID
Isoniazid (INH) acts on the cell wall of mycobacteria by inhibiting the synthesis of mycolic acid, a fatty acid found mainly in mycobacteria. Para-Aminosalicylic Acid (PAS) is an antimetabolite that inhibits the biosynthesis of folate or folic acid in mycobacteria the same way that the sulphonamides act in other pathogenic bacteria. Ethambutol inhibits the incorporation of mycolic acids into the mycobacterial cell wall. Rifampin (RIF) is a protein synthesis inhibitor in mycobacteria; and it is also effective against some pathogenic Gram positive bacteria and some Gram negative rods. Streptomycin like rifampin is a protein synthesis inhibitor.
BACTERIAL RESISTANCE TO ISONIAZID
Mycobacteria easily develop resistance to antimicrobial agents and the mycolic acid content of their cell wall is a formidable force that expels most antibiotics. MDR TB and XDR TB organisms now exist and they have compromised the effective treatment of TB infections. Mutation in the active sites or binding sites of anti-TB drugs on mycobacteria is one of the factors that mediate resistance in M. tuberculosis.
References
Ashutosh Kar (2008). Pharmaceutical Microbiology, 1st edition. New Age International Publishers: New Delhi, India.
Axelsen P. H (2002). Essentials of Antimicrobial Pharmacology. Humana Press, Totowa, NJ.
Balfour H. H (1999). Antiviral drugs. N Engl J Med, 340, 1255–1268.
Bean B (1992). Antiviral therapy: current concepts and practices. Clin Microbiol Rev, 5, 146–182.
Beck R.W (2000). A chronology of microbiology in historical context. Washington, D.C.: ASM Press.
Champoux J.J, Neidhardt F.C, Drew W.L and Plorde J.J (2004). Sherris Medical Microbiology: An Introduction to Infectious Diseases. 4th edition. McGraw Hill Companies Inc, USA.
Chemotherapy of microbial diseases. In: Chabner B.A, Brunton L.L, Knollman B.C, eds. Goodman and Gilman’s The Pharmacological Basis of Therapeutics. 12th ed. New York, McGraw-Hill; 2011.
Chung K.T, Stevens Jr., S.E and Ferris D.H (1995). A chronology of events and pioneers of microbiology. SIM News, 45(1):3–13.
Courvalin P, Leclercq R and Rice L.B (2010). Antibiogram. ESKA Publishing, ASM Press, Canada.
Denyer S.P., Hodges N.A and Gorman S.P (2004). Hugo & Russell’s Pharmaceutical Microbiology. 7th ed. Blackwell Publishing Company, USA. Pp.152-172.
Dictionary of Microbiology and Molecular Biology, 3rd Edition. Paul Singleton and Diana Sainsbury. 2006, John Wiley & Sons Ltd. Canada.
Drusano G.L (2007). Pharmacokinetics and pharmacodynamics of antimicrobials. Clin Infect Dis, 45(suppl):89–95.
Engleberg N.C, DiRita V and Dermody T.S (2007). Schaechter’s Mechanisms of Microbial Disease. 4th ed. Lippincott Williams & Wilkins, Philadelphia, USA.
Finch R.G, Greenwood D, Norrby R and Whitley R (2002). Antibiotic and chemotherapy, 8th edition. Churchill Livingstone, London and Edinburg.
Gillespie S.H and Bamford K.B (2012). Medical Microbiology and Infection at a glance. 4th edition. Wiley-Blackwell Publishers, UK.
Gordon Y. J, Romanowski E.G and McDermitt A M (2005). A review of antimicrobial peptides and their therapeutic potential as anti-infective drugs. Current Eye Research, 30(7): 505-515.
Hardman JG, Limbird LE, eds. Goodman and Gilman’s The Pharmacological Basis of Therapeutics. 10th ed. New York: McGraw-Hill; 2001.
Katzung, B. G. (2003). Basic and Clinical Pharmacology (9th ed.). NY, US, Lange.
Kontoyiannis D.P and Lewis R.E (2002). Antifungal drug resistance of pathogenic fungi. Lancet. 359:1135–1144.
Discover more from #1 Microbiology Resource Hub
Subscribe to get the latest posts to your email.


