Chloramphenicol is a protein synthesis inhibitor but the antibiotic unlike other drugs that interfere with bacterial protein biosynthesis (e.g. tetracycline and aminoglycosides) binds to the 50S ribosomal subunit of the target bacterial ribosome the same manner that macrolides (e.g. erythromycin) exhibit their antibacterial activity. Chloramphenicol and erythromycin exhibit similar modes of antibacterial action or activity on their target bacterial pathogen because they both bind to the 50S ribosomal subunit of the bacteria RNA.
Chloramphenicol, an inexpensive antibiotic has antibacterial activity against a wide variety of pathogenic bacteria including aerobic and anaerobic organisms; and the antibiotic is selectively toxic since it only binds to the 50S ribosomal subunit of bacteria. But the drug may have minimal effect on the eukaryotic mitochondria (especially those found in the bone marrow of humans), and this explains the reason why chloramphenicol is toxic and must be used with caution in some disease condition especially in pregnant women and infants.
SOURCES OF CHLORAMPHENICOL
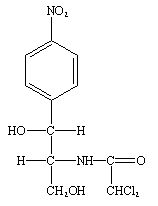
Chloramphenicol is a naturally synthesized antibiotic, and it is naturally biosynthesized by bacteria in the genus Streptomyces (particularly Streptomyces venezuelae). Chloramphenicol was first isolated in 1947 from a soil sample in Venezuela; and it is primarily synthesized by S. venezuelae. However, chloramphenicol is now synthesized by chemical processes in which the nitrobenzene ring of the antibiotic (Figure 1) is chemically modified.
STRUCTURE OF CHLORAMPHENICOL
Chloramphenicol has a simple chemical structure unlike the other antibiotics that are protein synthesis inhibitors (e.g. tetracyclines). The structure of chloramphenicol is a nitrobenzene ring (Figure 1). Chemical modification of the basic structure of chloramphenicol (i.e. the nitrobenzene ring) leads to the production of synthetic forms of the antibiotic.
CLINICAL APPLICATION OF CHLORAMPHENICOL
Clinically, chloramphenicol is the drug of choice for typhoid fever, bacterial meningitis and infectious diseases caused by some intracellular bacterial parasites such as Rickettsial species and Chlamydial species; but the toxicity of the antibiotic has restricted its clinical usage with exception in life-threatening situations where the antibiotic must be administered.
SPECTRUM OF ACTIVITY OF CHLORAMPHENICOL
Chloramphenicol has a broad spectrum of activity and it is effective against both pathogenic Gram-positive and Gram-negative bacteria. And the antibiotic is mainly bacteriostatic in action. However, chloramphenicol may be bacteriocidal to some pathogenic Gram negative bacteria including Streptococcus species, Neisseria species and Haemophilus species.
MECHANISM OR MODE OF ACTION OF CHLORAMPHENICOL
Chloramphenicol inhibits translation or protein synthesis in pathogenic bacteria by binding to the 50S ribosomal subunit; and this binding blocks the activities of peptidyl transferase which is mainly responsible for the elongation of polypeptide bonds during protein biosynthesis in bacteria. The interference of the activities of the enzyme, peptidyl transferase by chloramphenicol generally blocks peptide bond formation, and this also prevents the formation and/or elongation of the peptide chain during protein synthesis in the target bacteria. Chloramphenicol is bacteriostatic in action because it irreversibly binds the 50S ribosomal subunit of the bacterial ribosome; but the antibiotic can be bacteriocidal for some Gram negative bacteria.
BACTERIAL RESISTANCE TO CHLORAMPHENICOL
Bacterial resistance to chloramphenicol is usually mediated by plasmids. Chloramphenicol resistance may be carried on a bacterial plasmid that also codes for resistance to other drug classes such as tetracycline and ampicillin. The notable mechanisms of bacterial resistance to the antimicrobial onslaught of chloramphenicol include reduced membrane permeability, mutation of the 50S ribosomal subunit, and the elaboration of chloramphenicol acetyl-transferase. The target bacterial pathogen mutates the target site of the drug (i.e. the 50S ribosomal subunit of the RNA) and modifies its membrane so that the drug will have little or no penetration into the cell. Nevertheless, chloramphenicol is still active against some bacterial pathogens including some multidrug resistant bacteria; and this is attributed to the reduced or limited usage of the drug over some decades now.
PHARMACOKINETICS OF CHLORAMPHENICOL
Chloramphenicol is mainly administered orally, and the drug is well absorbed by the body via the gastrointestinal tract (GIT). Chloramphenicol is extremely lipid-soluble; and the drug has a large apparent volume of distribution in the body. It penetrates effectively into all tissues of the body including the brain. The antibiotic is excreted in its inactive form through the urine; and chloramphenicol penetrates the CSF and/or CNS effectively.
SIDE EFFECT/TOXICITY OF CHLORAMPHENICOL
Chloramphenicol is a toxic antibiotic, and its untoward effects which have generally compromised its clinical usage include suppression of bone marrow development and aplastic anaemia in patients. Other side effects include respiratory dysfunction and allergic reactions in the recipient host. Chloramphenicol is contraindicated in pregnant women and infants because of its notable toxicity.
References
Ashutosh Kar (2008). Pharmaceutical Microbiology, 1st edition. New Age International Publishers: New Delhi, India.
Axelsen P. H (2002). Essentials of Antimicrobial Pharmacology. Humana Press, Totowa, NJ.
Balfour H. H (1999). Antiviral drugs. N Engl J Med, 340, 1255–1268.
Bean B (1992). Antiviral therapy: current concepts and practices. Clin Microbiol Rev, 5, 146–182.
Beck R.W (2000). A chronology of microbiology in historical context. Washington, D.C.: ASM Press.
Champoux J.J, Neidhardt F.C, Drew W.L and Plorde J.J (2004). Sherris Medical Microbiology: An Introduction to Infectious Diseases. 4th edition. McGraw Hill Companies Inc, USA.
Chemotherapy of microbial diseases. In: Chabner B.A, Brunton L.L, Knollman B.C, eds. Goodman and Gilman’s The Pharmacological Basis of Therapeutics. 12th ed. New York, McGraw-Hill; 2011.
Chung K.T, Stevens Jr., S.E and Ferris D.H (1995). A chronology of events and pioneers of microbiology. SIM News, 45(1):3–13.
Courvalin P, Leclercq R and Rice L.B (2010). Antibiogram. ESKA Publishing, ASM Press, Canada.
Denyer S.P., Hodges N.A and Gorman S.P (2004). Hugo & Russell’s Pharmaceutical Microbiology. 7th ed. Blackwell Publishing Company, USA. Pp.152-172.
Dictionary of Microbiology and Molecular Biology, 3rd Edition. Paul Singleton and Diana Sainsbury. 2006, John Wiley & Sons Ltd. Canada.
Drusano G.L (2007). Pharmacokinetics and pharmacodynamics of antimicrobials. Clin Infect Dis, 45(suppl):89–95.
Engleberg N.C, DiRita V and Dermody T.S (2007). Schaechter’s Mechanisms of Microbial Disease. 4th ed. Lippincott Williams & Wilkins, Philadelphia, USA.
Finch R.G, Greenwood D, Norrby R and Whitley R (2002). Antibiotic and chemotherapy, 8th edition. Churchill Livingstone, London and Edinburg.
Gillespie S.H and Bamford K.B (2012). Medical Microbiology and Infection at a glance. 4th edition. Wiley-Blackwell Publishers, UK.
Hardman JG, Limbird LE, eds. Goodman and Gilman’s The Pharmacological Basis of Therapeutics. 10th ed. New York: McGraw-Hill; 2001.
Katzung, B. G. (2003). Basic and Clinical Pharmacology (9th ed.). NY, US, Lange.
Kontoyiannis D.P and Lewis R.E (2002). Antifungal drug resistance of pathogenic fungi. Lancet. 359:1135–1144.
Discover more from #1 Microbiology Resource Hub
Subscribe to get the latest posts to your email.


