Gram staining is a general purpose bacteriological identification technique used in the bacteriology section of the microbiology laboratory to identify and differentiate bacteria into two groups i.e. Gram-positive and Gram-negative. It was discovered by Christian Gram (1853-1938), a Danish scientist in 1884. Christian Gram showed that the cells of some bacteria could be easily decolorized with organic solvents (e.g. ethanol) after staining with some basic dyes (e.g. crystal violet) while the cells of other group of bacteria (known as Gram-positive bacteria) resisted the decolorization after the staining process.
Gram staining technique is generally used to identify bacterial pathogens from cultures and clinically important specimens based on their Gram reaction. Bacteria that are neither Gram-positive nor Gram-negative are termed Gram-variable organisms and such bacteria cannot be identified with the Gram staining technique. Gram variable bacteria are usually old bacterial cultures that have lost their cell wall structure over time. Gram indeterminate bacteria are those bacteria that cannot be detected by Gram staining technique.
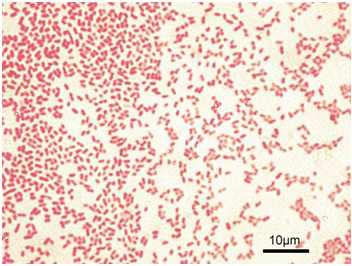
Typical examples of Gram indeterminate bacterium are Mycobacterium species that possess a waxy/lipid-containing cell wall (that contains mycolic acid).In the Gram staining technique, Gram-positive bacteria stain purple or blue-violet (Figure 1) while Gram-negative bacteria stain red or pink (Figure 2). Gram staining is one of the most important differential staining techniques used by microbiologists worldwide to characterize bacteria based on their cell wall compositions. Crystal violet (primary stain), Lugol’s iodine (mordant), ethanol/acetone (decolorizer) and safranin (secondary or counter stain) are the stains or dyes used for carrying out the Gram staining technique in the microbiology laboratory.
Gram-positive bacteria retain the primary stain after decolorization while Gram-negative bacteria do not. The mordant (iodine) increases the interaction of the bacterial cell wall with the primary stain while the decolorizer removes the primary stain from the cell wall of the test bacteria (i.e. if the organism is Gram-negative). Gram-negative bacteria retain the colour of the counter stain (safranin), and thus appear reddish or pinkish under the microscope.

PROTOCOL FOR GRAM STAINING TECHNIQUE
- Prepare a thin smear of the test bacteria on a clean glass slide.
- Allow the prepared smear to dry while standing on a staining rack.
- Add crystal violet stain using a dropper. Allow for 60 seconds.
- Wash off the stain with clean water.
- Add or cover the smear with Lugol’s iodine using a dropper. Allow for 60 seconds.
- Wash off the stain with clean water.
- Add a drop of acetone or ethanol to decolorize the stained smear.
- Wash off with water immediately.
- Add safranin to the stained smear. Allow for 60 seconds or a maximum of 2 minutes.
- Wash off the stain with clean water. Allow to dry while standing on the staining rack. Ensure to carefully clean the back of the stained slide with a clean cloth or cotton wool.
- Add a drop of immersion oil on the stained smear.
- View the stained smear under the microscope using the oil immersion objective lens (100x).
- Gram-positive bacteria appear blue-violet under the microscope while Gram-negative bacteria appear red. Ensure to take notice of the bacteria shape (i.e. cocci, rod, coccobacilli or streptococci) when reporting the result of a Gram stained smear.
References
Basic laboratory procedures in clinical bacteriology. World Health Organization (WHO), 1991. Available from WHO publications, 1211 Geneva, 27-Switzerland.
Beers M.H., Porter R.S., Jones T.V., Kaplan J.L and Berkwits M (2006). The Merck Manual of Diagnosis and Therapy. Eighteenth edition. Merck & Co., Inc, USA.
Biosafety in Microbiological and Biomedical Laboratories. 5th edition. U.S Department of Health and Human Services. Public Health Service. Center for Disease Control and Prevention. National Institute of Health. HHS Publication No. (CDC) 21-1112.2009.
Cheesbrough M (2010). District Laboratory Practice in Tropical Countries. Part I. 2nd edition. Cambridge University Press, UK.
Cheesbrough M (2010). District Laboratory Practice in Tropical Countries. Part 2. 2nd edition. Cambridge University Press, UK.
Collins C.H, Lyne P.M, Grange J.M and Falkinham J.O (2004). Collins and Lyne’s Microbiological Methods. Eight edition. Arnold publishers, New York, USA.
Disinfection and Sterilization. (1993). Laboratory Biosafety Manual (2nd ed., pp. 60-70). Geneva: WHO.
Garcia L.S (2010). Clinical Microbiology Procedures Handbook. Third edition. American Society of Microbiology Press, USA.
Garcia L.S (2014). Clinical Laboratory Management. First edition. American Society of Microbiology Press, USA.
Fleming, D. O., Richardson, J. H., Tulis, J. I. and Vesley, D. (eds) (1995). Laboratory Safety: Principles and practice. Washington DC: ASM press.
Dubey, R. C. and Maheshwari, D. K. (2004). Practical Microbiology. S.Chand and Company LTD, New Delhi, India.
Gillespie S.H and Bamford K.B (2012). Medical Microbiology and Infection at a glance. 4th edition. Wiley-Blackwell Publishers, UK.
Discover more from #1 Microbiology Resource Hub
Subscribe to get the latest posts to your email.


