Bacterial smear is defined as a dehydrated or dried preparation of a bacterial suspension (cells) on a clean glass slide. The source of bacteria for the preparation or making of a smear can be from an agar slant, broth (liquid) culture and from a culture plate.
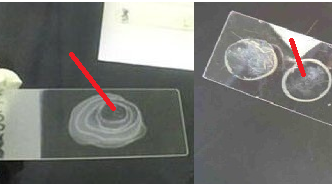
A smear is simply a bacterial preparation made on a clean glass slide (Figure 1).
A well prepared bacterial smear must possess some qualities as follows:
- The shape of the bacterial cell will not be distorted or unclear during microscopy.
- The bacteria will be properly and evenly separated from one another after spreading out on the glass slide and subsequent microscopy.
- During staining of the prepared smear, the bacteria will not be washed off the slide.
PROCEDURE OF MAKING A BACTERIAL SMEAR
Bacterial smear can be made using the following protocol:
- Using a grease pencil, mark one end of the slide with the name of the bacterial culture.
- Transfer a speck of the bacteria to a drop of normal saline on a clean glass slide. If the bacteria is from a liquid medium (i.e. broth), place 1-2 drops or loops of bacteria on a clean glass slide.
- Spread out the bacteria (from a broth or solid culture) mixture over a given surface area on the glass slide (Figure 1). Always ensure that a thin preparation is made and, that a space is left on each of the four sides of the glass slide for proper viewing under the microscope.
- Air dry the glass slide at room temperature (25-28oC) while ensuring that it is free from dust.
- Heat-fix the smear by passing it through a Bunsen burner flame three times or by flooding the smear with 70 % methanol or ethanol and allowed for 2 min. Always ensure that the prepared smear is on top of the glass slide as you pass it through the Bunsen burner flame. Over-heating the smear on the slide will cause the organism to char or burn; and this will affect the cell structure of the organism – thus giving you a bad test result during staining and viewing.
- After heat-fixing, the fixed smear can be stained on a staining rack using different types of dyes depending on the staining technique used. There are different staining techniques that are available in the bacteriology laboratory.
The staining can be simple or differential. Some of the staining techniques used in the bacteriology laboratory including simple staining and differential staining technique include:
- Gram staining: For dividing bacteria into Gram positive and Gram negative organism.
- Ziehl-Neelsen staining: For detecting acid-fast bacilli e.g. Mycobacterium species.
- Albert staining: For detecting Corynebacterium diphtheria from sputum specimen.
- Wayson staining: For detecting Yersinia pestis
- Loeffler methylene blue stain: For detection of Bacillus anthracis
- Malachite green staining: For detection of bacterial endospores.
- Crystal violet staining: For detection of bacterial spores.
- Robinow’s staining: For detection of bacterial DNA.
- Capsule staining: For detection of bacterial capsules.
After preparing the smear and staining it, add a drop of immersion oil on the stained slide, and examine or view the slide under the microscope using the oil immersion objective lens (100x).
HEAT FIXING
Heat-fixing is defined as the microbiological technique of attaching bacterial cells onto the surface of a glass slide by the use of a Bunsen burner flame or 70% of methanol. A gentle heating of a bacterial cell on a slide when passed through the blue flame of a Bunsen burner actually kills the bacteria but nevertheless, heat-fixing does not distort the cell structure of the organism. The cell structure of the bacterial cell becomes distorted if and only when the slide is over-heated. Heat-fixing of bacterial cells allows the internal and external structures of a microorganism to be intact during staining and microscopy.
References
Basic laboratory procedures in clinical bacteriology. World Health Organization (WHO), 1991. Available from WHO publications, 1211 Geneva, 27-Switzerland.
Beers M.H., Porter R.S., Jones T.V., Kaplan J.L and Berkwits M (2006). The Merck Manual of Diagnosis and Therapy. Eighteenth edition. Merck & Co., Inc, USA.
Biosafety in Microbiological and Biomedical Laboratories. 5th edition. U.S Department of Health and Human Services. Public Health Service. Center for Disease Control and Prevention. National Institute of Health. HHS Publication No. (CDC) 21-1112.2009.
Cheesbrough M (2010). District Laboratory Practice in Tropical Countries. Part I. 2nd edition. Cambridge University Press, UK.
Cheesbrough M (2010). District Laboratory Practice in Tropical Countries. Part 2. 2nd edition. Cambridge University Press, UK.
Collins C.H, Lyne P.M, Grange J.M and Falkinham J.O (2004). Collins and Lyne’s Microbiological Methods. Eight edition. Arnold publishers, New York, USA.
Disinfection and Sterilization. (1993). Laboratory Biosafety Manual (2nd ed., pp. 60-70). Geneva: WHO.
Garcia L.S (2010). Clinical Microbiology Procedures Handbook. Third edition. American Society of Microbiology Press, USA.
Garcia L.S (2014). Clinical Laboratory Management. First edition. American Society of Microbiology Press, USA.
Fleming, D. O., Richardson, J. H., Tulis, J. I. and Vesley, D. (eds) (1995). Laboratory Safety: Principles and practice. Washington DC: ASM press.
Dubey, R. C. and Maheshwari, D. K. (2004). Practical Microbiology. S.Chand and Company LTD, New Delhi, India. Gillespie S.H and Bamford K.B (2012). Medical Microbiology and Infection at a glance. 4th edition. Wiley-Blackwell Publishers, UK.
Discover more from #1 Microbiology Resource Hub
Subscribe to get the latest posts to your email.