Cephalosporins are beta-lactam antibiotics that are penicillinase-resistant, and with related mode of action to the penicillins. Drugs in this category are clinical substitutes for penicillins due to the development of resistance to the later by pathogenic bacteria. Cephalosporins are classified into four (4) generations based on their spectrum of activity, side chain modifications and clinical applications.
FIRST GENERATION CEPHALOSPORINS
The 1st-generation cephalosporins are active against Gram positive bacteria, and they include cephalexin, cephalothin, cephapirin, cefazolin, cephadrine and cefadrox. They have a narrow-spectrum of activity against pathogenic bacteria especially the Gram negative rods (e.g. Klebsiella species and Escherichia coli). Though their activity is mainly against penicillinase-resistant Gram positive bacteria, antibiotics in this group are also active against some Enterobacteriaceae.
SECOND GENERATION CEPHALOSPORINS
The 2nd-generation cephalosporins were designed to counter the resistance of Gram negative bacteria (mediated by beta-lactamase production). They have expanded antibacterial activity against pathogenic bacteria in the family Enterobacteriaceae (e.g. Klebsiella species, Haemophilus species and E. coli)and even against some anaerobic bacteria (e.g. Bacteroides species). Antibiotics that are 2nd-generation cephalosporins include cefaclor, cefotetan, cefuroxime, and cefoxitin. 2nd-generation cephalosporins generally have expanded Gram negative activity than the 1st-generation cephalosporins that are more susceptible to beta-lactamase enzymes secreted by these pathogens.
THIRD GENERATION CEPHALOSPORINS
The 3rd-generation cephalosporins include ceftazidime, cefotaxime, and ceftriaxone. Antibiotics in this category are more effective against Gram negative rods than the 2nd-generation cephalosporins; and they have antibacterial activity against a wide variety of Enterobacteriaceae and non-enteric bacteria (e.g. Pseudomonas aeruginosa). 3rd-generation cephalosporins have enhanced systemic activity against a wide variety of Gram negative rods including those that penetrate the CNS (e.g. Neisseria meningitidis); and they are the preferred antibiotic of choice in life-threatening bacterial diseases or infections of yet unknown cause (i.e. infections in which the causative agents are yet to be isolated from patients specimen in the microbiology laboratory).
FOURTH GENERATION CEPHALOSPORINS
The 4th-generation cephalosporins have extended antibacterial spectrum or activity against pathogenic bacteria (especially the Gram negative rods) to which the 3rd-generation cephalosporins are least effective. They include cefepime and cefpirome. 4th-generation cephalosporins have activity against Enterobacter species, Citrobacter species, Neisseria species, Haemophilus species, Enterobacteriaceae and a wide variety of Gram negative bacteria. Antibiotics in this category have more OM-penetrating potential than the other generations of cephalosporins; and they are more stable to beta-lactamase enzymes secreted by Gram negative bacteria.
FIFTH GENERATION CEPHALOSPORINS
The 5th-generation cephalosporins are usually administered intravenously (IV). And they are active against a variety of multidrug resistant bacteria pathogens including the dreaded methicillin resistant Staphylococcus aureus (MRSA). The 5th-generation cephalosporins include Ceftaroline Fosamil (IV) and Ceftobiprole (IV).
SOURCES OF CEPHALOSPORINS
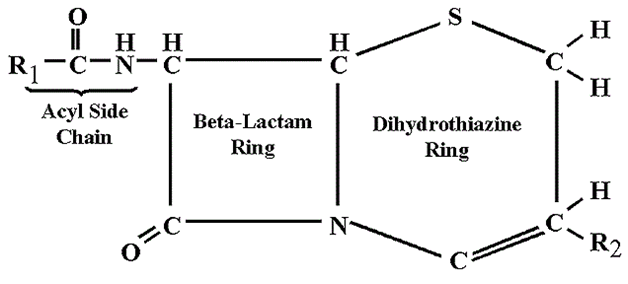
Cephalosporins are naturally sourced from the fungus Cephalosporium (e.g. C. acremonium). The cephalosporins were originally produced in the early 1940s from moulds (i.e. Cephalosporium species). However, cephalosporins can also be produced semi-synthetically by the addition of substituent groups to the side chains of the 7-aminocephalosporanic acid structure (Figure 1).
STRUCTURE OF CEPHALOSPORINS
The basic structure of the cephalosporins is known as 7-aminocephalosporanic acid (Figure 1). Addition of substituent groups to this basic structure result in the production of different variants of cephalosporins.
CLINICAL APPLICATION OF CEPHALOSPORINS
Clinically, the cephalosporins are effective against a wide variety of pathogenic bacteria especially the Gram negative rods to which the penicillins have little or no activity against. 1st-generation cephalosporins are very active against Gram positive bacteria (e.g. Staphylococcus species). 2nd-generation cephalosporins are effective against Gram negative rods (e.g. Klebsiella species and Proteus species) but not against Pseudomonas species. 3rd-generation cephalosporins are active against Pseudomonas species and a variety of Enterobacteriaceae and other Gram negative rods. 4th-generation cephalosporins are active against a variety of pathogenic bacteria including those that are resistant to the antibacterial onslaught of 3rd-generation cephalosporins.
SPECTRUM OF ACTIVITY OF CEPHALOSPORINS
Cephalosporins (including the 1st, 2nd, 3rd and 4th-generations) have a broader spectrum of activity, and they are bacteriocidal in action. They have activity against both Gram positive and Gram negative bacteria.
MECHANISM OR MODE OF ACTION OF CEPHALOSPORINS
Cephalosporins like the penicillins are cell wall synthesis inhibitors. They bind to the PBPs on bacterial cell wall, and interfere with the synthesis of peptidoglycan in growing bacteria. Thus, cephalosporins have similar mode of action like the penicillins but the former have increased stability to beta-lactamases produced by Gram negative bacteria than the later.
BACTERIAL RESISTANCE TO CEPHALOSPORINS
Though very effective against a wide variety of pathogenic bacteria, most cephalosporins are fast becoming susceptible to some antibiotic-degrading enzymes such as the ESBLs and other multidrug resistance factors produced by bacteria. The production of ESBLs aside other antibiotic-hydrolyzing enzymes by Gram negative bacteria have significantly compromised the clinical use of the cephalosporins for treating some bacterial-related infections or diseases.
PHARMACOKINETICS OF CEPHALOSPORINS
Cephalosporins are generally well tolerated by the body when administered orally or otherwise. They have a wider systemic distribution in the body.
SIDE EFFECT/TOXICITY OF CEPHALOSPORINS
Untoward effects due to the usage of cephalosporins are attributed to the host’s allergic reactions to the drug as is applicable to the use of penicillins. But hypersensitivity is fewer compared to that produced by penicillins.
References
Ashutosh Kar (2008). Pharmaceutical Microbiology, 1st edition. New Age International Publishers: New Delhi, India.
Axelsen P. H (2002). Essentials of Antimicrobial Pharmacology. Humana Press, Totowa, NJ.
Balfour H. H (1999). Antiviral drugs. N Engl J Med, 340, 1255–1268.
Bean B (1992). Antiviral therapy: current concepts and practices. Clin Microbiol Rev, 5, 146–182.
Beck R.W (2000). A chronology of microbiology in historical context. Washington, D.C.: ASM Press.
Champoux J.J, Neidhardt F.C, Drew W.L and Plorde J.J (2004). Sherris Medical Microbiology: An Introduction to Infectious Diseases. 4th edition. McGraw Hill Companies Inc, USA.
Chemotherapy of microbial diseases. In: Chabner B.A, Brunton L.L, Knollman B.C, eds. Goodman and Gilman’s The Pharmacological Basis of Therapeutics. 12th ed. New York, McGraw-Hill; 2011.
Chung K.T, Stevens Jr., S.E and Ferris D.H (1995). A chronology of events and pioneers of microbiology. SIM News, 45(1):3–13.
Courvalin P, Leclercq R and Rice L.B (2010). Antibiogram. ESKA Publishing, ASM Press, Canada.
Denyer S.P., Hodges N.A and Gorman S.P (2004). Hugo & Russell’s Pharmaceutical Microbiology. 7th ed. Blackwell Publishing Company, USA. Pp.152-172.
Dictionary of Microbiology and Molecular Biology, 3rd Edition. Paul Singleton and Diana Sainsbury. 2006, John Wiley & Sons Ltd. Canada.
Drusano G.L (2007). Pharmacokinetics and pharmacodynamics of antimicrobials. Clin Infect Dis, 45(suppl):89–95.
Discover more from Microbiology Class
Subscribe to get the latest posts sent to your email.