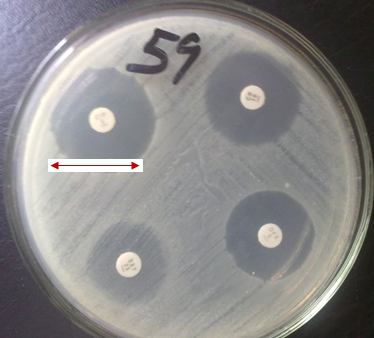
When a pathogenic microorganism is isolated from the specimen of a patient, the clinical microbiology laboratory will often carry out in vitro antimicrobial susceptibility testing (antibiogram) on the isolated pathogen in order to determine the antimicrobial susceptibility profile of the microbe to a range of antibiotics/drugs using antibiotic susceptibility disks, so that treatment can be appropriately guided.
Drug or antimicrobial susceptibility testing (AST) helps to provide both the physician and microbiologist with reliable data that can confidently predict the in vivo effectiveness/efficacy of the drug in question. This procedure when undertaken is very essential to proper therapy and adequate management of the patient. Antimicrobial drug susceptibility testing can show which drugs are most effective against a given pathogen, and it also helps to give an estimate of the adequate therapeutic dose required to fight the pathogen.It guides physicians in the choice of appropriate drugs/antibiotics for therapy. Susceptibility testing in the clinical microbiology laboratory is usually undertaken based on diffusion or dilution techniques.
The diffusion techniques makes use of paper disk impregnated with antimicrobial agents, while the dilution techniques usually make use of the powdered form of the drug in question that is serially diluted and tested on a given pathogen in order to determine the minimum inhibitory concentration (MIC) of the agent. The disk diffusion technique measures the zone of inhibition of microbial growth caused by the drug in millimeters (mm). Regular antimicrobial susceptibility tests in the clinical microbiology laboratory is crucial because it helps to spot and control resistant microbes, give an up-to-date information on the best drug for empirical treatment and routine antibiogram for suspect pathogens helps to prevent the emergence and spread of resistant genes in an hospital environment.
Antibiogram is the pattern of sensitivities of a given pathogenic microorganism towards an array of antibiotics (that are usually impregnated in a paper disk). The term “antibiogram” can be used synonymously with antimicrobial susceptibility testing (AST). Antibiogram helps the microbiologist or clinician to forecast the possibility of treating an infectious disease successfully using a particular drug or an array of antimicrobial agent; and it prevents the emergence and spread of drug resistant microorganisms within the hospital and community because the results of AST guides the physician or health personnel on the choice of antimicrobial therapy to proffer for the sick patient.
Antimicrobial susceptibility testing should not be carried out on commensals, contaminants or normal flora of the host patients including organisms that has no relationship whatsoever with the disease process of the patient. This is why it is critical to do a proper identification of the isolated organism in order to ensure that they are not commensals but pathogens responsible for the disease process. Susceptibility testing should only be evaluated on pathogens or pure cultures of microorganisms that have been considered to be causing the infectious disease, and this can only be actualized if the proper identification and characterization tests are carried out – in a bid to isolating only pure cultures from the patient’s specimen. Antibiogram will be misleading or useless to perform if adequate identification tests are not carried prior to the susceptibility test proper.
NOTE: Therapeutic dose is defined as the drug level that is required for clinical treatment of a particular infection.
The goal of antimicrobial drug susceptibility testing is to predict the in vivo success or failure of antibiotic therapy. Antimicrobial drug susceptibility testing which also means sensitivity tests, are usually performed in vitro, and the growth response of an isolated pathogen to a particular drug or drugs is measured. Sensitivity tests are performed under standardized conditions so that the results are reproducible. The test results when combined with clinical information and experience should be used to guide antibiotic choice, in order to select the most appropriate drug that will be most efficacious against a given pathogen.
Laboratory tests for antimicrobial drug susceptibility testing are often performed in one of the following scenarios:
- In infections where eradication of the infecting pathogen require drugs that are rapidly cidal (killing).
- When a drug – resistant pathogen is isolated.
- When the infection is fatal unless treated specifically with the right type of drugs.
It is a fact and truth that when a particular pathogen has been isolated and identified as the sole causative agent of a particular infection, clinicians/physicians often select drugs for treatment based on prior or current clinical experiences with certain infections and pathogens. Nevertheless, antimicrobial drug susceptibility testing should not be overlooked or underrated in a clinical setting so as not to encourage the emergence of drug – resistant strains which often emanate from the misuse of drugs.
In addition to this, antimicrobial drug susceptibility testing:
- Encourages rational selection of antimicrobial agents.
- Determines drug of choice for a particular infection.
- Determines drugs to be used in synergy, especially in the treatment of mixed infections.
- Determine the susceptibility of a given pathogen to known concentrations of a drug.
- Determine the potency of antimicrobial agents in solutions.
- Determine the concentration of a given drug in body fluids.
- Discourages the development of drug – resistant strains of microbes, because when drugs are appropriately administered in the right concentration and dosage, and for the right type of infection at the right time; it will be very difficult for drug – resistant strains of pathogens to emerge.
It is noteworthy that antimicrobial drug susceptibility testing should never be performed on commensal organisms or contaminants because this would mislead the physician in administering antimicrobial agents/drugs to the patient. This could result to the patient receiving ineffective and unnecessary antimicrobial therapy, causing possible untoward effects in the patient, and resistance of the administered drug to other potentially pathogenic organisms.
References
Arora D.R (2004). Quality assurance in microbiology. Indian J Med Microbiol, 22:81-86.
Ashutosh Kar (2008). Pharmaceutical Microbiology, 1st edition. New Age International Publishers: New Delhi, India.
Barenfanger J, Drakel C and Kacich (1999). Clinical and Financial Benefits of Rapid Bacterial Identification and Antimicrobial Susceptibility Testing. Journal of Clinical Microbiology, 37(5):1415-1418.
Denyer S.P., Hodges N.A and Gorman S.P (2004). Pharmaceutical Microbiology. 7th ed. Blackwell Publishing Company, USA.
Doern G, Brueggemannn A.B, Perla R, Daly D, Halkias D, Jones R.N, Saubolle M.A (1997). Multicenter laboratory evaluation of the bioMerieux Vitek antimicrobial susceptibility testing system with 11 antimicrobial agents versus members of the family Enterobacteriaceae and Pseudomonas aeruginosa. J Clin Microbiol, 35:2115–2119.
Doern G, Vautour R, Gaudet M, Levy B (1994). Clinical impact of rapid in vitro susceptibility testing and bacterial identification. J Clin Microbiol, 32:1757–1762.
Doern G.V (1995). Susceptibility tests of fastidious bacteria. Manual of Clinical Microbiology, 6th edition, Murray P.R, Baron E.J, Pfaller M.A, Tenover F.C, Yolken R, American Society for Microbiology, Washington DC, Pp. 1342-1349.
Funke G, Monnet D, deBernardis C, von Graevenitz A, Freney J (1998). Evaluation of the VITEK 2 system for rapid identification of medically relevant Gram-negative rods. J Clin Microbiol, 36:1948–1952.
Garcia L.S (2010). Clinical Microbiology Procedures Handbook. Third edition. American Society of Microbiology Press, USA.
Hart C.A (1998). Antibiotic Resistance: an increasing problem? BMJ, 316:1255-1256.
Livermore D.M, Winstanley T.B, Shannon K.P (2001). Interpretative reading: recognizing the unusual and inferring resistance mechanisms from resistance phenotypes.
J Antimicrob Chemother, 48 Suppl 1:87-102.
Madigan M.T., Martinko J.M., Dunlap P.V and Clark D.P (2009). Brock Biology of Microorganisms, 12th edition. Pearson Benjamin Cummings Inc, USA.
Mahon C. R, Lehman D.C and Manuselis G (2011). Textbook of Diagnostic Microbiology. Fourth edition. Saunders Publishers, USA.
National Committee for Clinical Laboratory Standards. Performance Standards for antimicrobial susceptibility testing. 8th Informational Supplement. M100 S12. National Committee for Clinical Laboratory Standards, 2002. Villanova, Pa.
Washington J.A (1993). Rapid antimicrobial susceptibility testing: technical and clinical considerations. Clin Microbiol Newsl, 15:153–155.
Discover more from #1 Microbiology Resource Hub
Subscribe to get the latest posts to your email.