The output of an antimicrobial drug susceptibility test is often expressed as raw data which are either expressed in the form of a zone size/diameter or minimum inhibitory concentration (MIC). The Clinical Laboratory Standard Institute (CLSI) formerly known as National Committee for Clinical Laboratory Standard (NCCLS) has published interpretation criteria for different antimicrobial agents. This criteria guide clinical microbiologists in the reporting and interpretation of antimicrobial drug susceptibility test results.
Based on these criteria, antimicrobial drug susceptibility tests results can be reported out in a variety of ways.
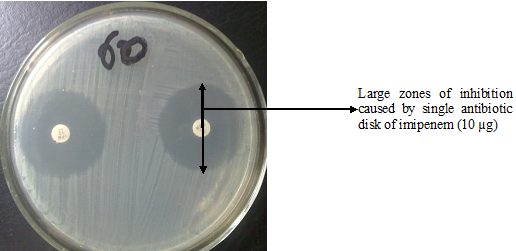
Susceptible: The “susceptible category” implies that microorganisms are inhibited by the usually achievable concentrations of antimicrobial agent when the recommended dosage is used for the site of infection. This is not in any way definitive or final as chances for clinical success in the use of any particular drug that shows reasonable efficacy against a pathogen in vitro. However, the result of an in vitro susceptibility test as exemplified by the zones of inhibition produced by the test antibiotic (Figure 1) is a clue to whether the test agent (i.e. the drug) will be effective for treating the disease or not. The imprecision in predicting clinical outcome based on susceptibility testing is due to the effect of the host response, site of infection, toxin production by the infecting organism that is independent of antimicrobial susceptibility, the presence of biofilm, drug pharmacodynamics, and other predisposing factors that affect antimicrobial drug susceptibility testing. Though very important in the management of an infection, it is not putative therefore, that an antimicrobial agent which kills or inhibit the growth of a pathogen in vitro will be very successful or efficacious in treatment. However, a pathogenic microorganism whose antimicrobial susceptibility test results false in this category implies that the test drug could be used to effectively inhibit the growth of the organism.
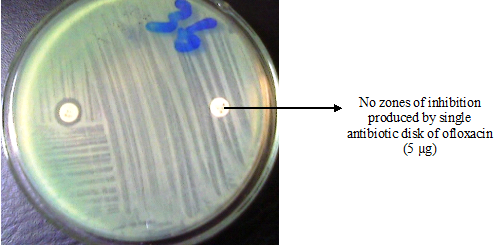
Resistant: The “resistant category” implies that microorganisms are not inhibited by the usually achievable concentrations of the agent with normal dosage schedules. It also means that the pathogen and not the patient is resistant to the drug, thus the drug has no killing power or potency towards the infecting pathogen. When a bacterial pathogen is resistant to a given drug either in vivo or in vitro, the test agent (i.e. the antibiotic) will be ineffective in causing any meaningful zones of inhibition when the pathogen is subjected to the antibiotic in an in vitro susceptibility test (Figure 2). This is not final as their exist some imprecision as mentioned above which can serve as impediment to its usefulness. However, with the exception of urinary bladder infections and some mycobacterial infections (e.g. TB), most physicians avoid the use of a drug classified as being “resistant” to treat infections.
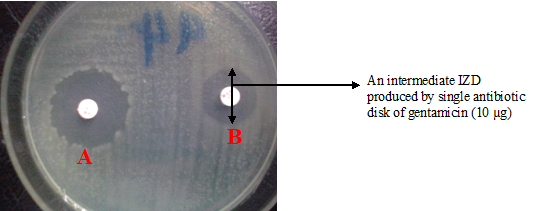
Intermediate: The “intermediate category” includes microorganisms with zone sizes or MIC’s that fall in between the ‘susceptible’ and ‘resistant’ category. Such microorganisms under this category approach usually attainable blood and tissue levels and their response rate to the antimicrobial agent is often lower than that of the ‘susceptible’ category. The intermediate category implies clinical efficacy in body sites where the drugs are physiologically concentrated or when a higher than normal dosage of a drug can be used. Thus, pathogenic microorganisms whose antimicrobial susceptibility results false in the “intermediate category” indicate that the organisms could be successfully treated with higher doses of the tested drug. Pathogenic bacteria whose inhibition zone diameter (IZD) falls between the “susceptible” and “resistant” line are generally termed “intermediate” even though the test drug produced some appreciable level of inhibition in vitro (Figure 3).
In summary, Susceptible indicates that the antimicrobial agent in question may be an appropriate choice for treating the infection or disease condition caused by the test organism. At the “susceptible” level, bacterial resistance is absent or at a clinically insignificant level; and thus the test antimicrobial agent is most suitable for treating the disease condition in the host.
Intermediate indicates a number of possibilities as follows:
- The potential utility of the antimicrobial agent in body sites where it may be concentrated (e.g., the urinary tract) or if high concentrations of the drug are used.
- Possible effectiveness of the antimicrobial agent against the isolate, but possibly less so than against a susceptible isolates.
- Use as an interpretive safety margin to prevent relatively small changes in test results from leading to major swings in interpretive category (e.g., resistant to susceptible or vice versa)
Resistant indicates that the antimicrobial agent in question may not be an appropriate choice for the treatment of a disease condition caused by the test pathogen. This is because the test organism is not inhibited with serum-achievable levels of the antimicrobial agent in question. Secondly, it could be because the test result highly correlates with a resistance mechanism (inherent or acquired by the test pathogen) that indicates questionable successful treatment.
References
Arora D.R (2004). Quality assurance in microbiology. Indian J Med Microbiol, 22:81-86.
Ashutosh Kar (2008). Pharmaceutical Microbiology, 1st edition. New Age International Publishers: New Delhi, India.
Barenfanger J, Drakel C and Kacich (1999). Clinical and Financial Benefits of Rapid Bacterial Identification and Antimicrobial Susceptibility Testing. Journal of Clinical Microbiology, 37(5):1415-1418.
Denyer S.P., Hodges N.A and Gorman S.P (2004). Pharmaceutical Microbiology. 7th ed. Blackwell Publishing Company, USA.
Doern G, Brueggemannn A.B, Perla R, Daly D, Halkias D, Jones R.N, Saubolle M.A (1997). Multicenter laboratory evaluation of the bioMerieux Vitek antimicrobial susceptibility testing system with 11 antimicrobial agents versus members of the family Enterobacteriaceae and Pseudomonas aeruginosa. J Clin Microbiol, 35:2115–2119.
Doern G, Vautour R, Gaudet M, Levy B (1994). Clinical impact of rapid in vitro susceptibility testing and bacterial identification. J Clin Microbiol, 32:1757–1762.
Doern G.V (1995). Susceptibility tests of fastidious bacteria. Manual of Clinical Microbiology, 6th edition, Murray P.R, Baron E.J, Pfaller M.A, Tenover F.C, Yolken R, American Society for Microbiology, Washington DC, Pp. 1342-1349.
Funke G, Monnet D, deBernardis C, von Graevenitz A, Freney J (1998). Evaluation of the VITEK 2 system for rapid identification of medically relevant Gram-negative rods. J Clin Microbiol, 36:1948–1952.
Garcia L.S (2010). Clinical Microbiology Procedures Handbook. Third edition. American Society of Microbiology Press, USA.
Hart C.A (1998). Antibiotic Resistance: an increasing problem? BMJ, 316:1255-1256.
Livermore D.M, Winstanley T.B, Shannon K.P (2001). Interpretative reading: recognizing the unusual and inferring resistance mechanisms from resistance phenotypes.
J Antimicrob Chemother, 48 Suppl 1:87-102.
Madigan M.T., Martinko J.M., Dunlap P.V and Clark D.P (2009). Brock Biology of Microorganisms, 12th edition. Pearson Benjamin Cummings Inc, USA.
Mahon C. R, Lehman D.C and Manuselis G (2011). Textbook of Diagnostic Microbiology. Fourth edition. Saunders Publishers, USA.
National Committee for Clinical Laboratory Standards. Performance Standards for antimicrobial susceptibility testing. 8th Informational Supplement. M100 S12. National Committee for Clinical Laboratory Standards, 2002. Villanova, Pa.
Washington J.A (1993). Rapid antimicrobial susceptibility testing: technical and clinical considerations. Clin Microbiol Newsl, 15:153–155.
Discover more from #1 Microbiology Resource Hub
Subscribe to get the latest posts to your email.


