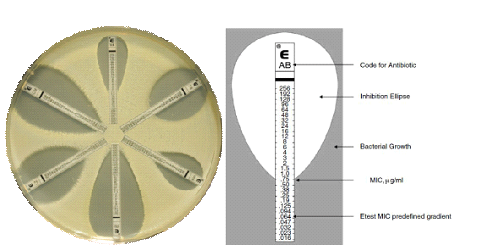
Over the past 70 years, antibiotics have saved countless number of lives across the globe and enabled the further development of modern medicine as well as antimicrobial agents of diverse types with putative activity against pathogenic microorganisms. Despite the current state of antimicrobial resistance (AMR) globally, these antibiotics are currently used to counter the nefarious (clinical and health challenges) of pathogenic microorganisms in both human and animal, as well as plant populations and the general environment. Scientists at the time (i.e., the pre-antibiotic era) were striving for a ‘magic bullet’ that could rid the body of infecting organisms or pathogens without harming the patient, a concept still attempted for, today.
Prior to the advent of antibiotics (microbial or chemical products or substances, which in low concentration have the ability to kill or inhibit the growth of microorganisms in the body), man suffered from diverse illnesses and medical conditions that lacked the appropriate antibiotics or antimicrobial agent to either treat or manage it. Clinically, antibiotics should only be toxic to their target pathogens, and not show any (uncontrollable harm) to the patient taking it. This implies that antibiotics should be selective toxic in order to be clinically relevant and safe for public usage.
Though the use, underuse, overuse and outright abuse of available antibiotics have contributed to the evolution and spread of antimicrobial resistance (AMR) globally, the discovery and development of antibiotics have contributed a great deal in the extension of human lifespan because of their lethal and selective action against pathogenic microorganisms either in vivo or in vitro. Prior to the discovery of antibiotics, mankind attributed disease or infections to some spiritual occurrence that afflicted man. At that time, many did not know that some microorganisms (e.g., pathogenic bacteria) where responsible for the infections or disease that afflicted man, animals and plants.
The use of antibiotic-producing microbes to prevent and treat infectious disease stretches back millennia, with traditional poultices of mouldy bread being used to treat open wounds in Serbia, China, Greece and Egypt more than 2000 years ago. Various moulds and plant extracts were used to treat microbial infections and diseases by some of the earliest civilizations. For example, the ancient Egyptians applied mouldy bread (i.e., spoiled bread contaminated by a type of microorganism called mould) to infected wounds in order to achieve healing. Previously, pneumonia and diarrhoea – that are caused by bacteria, were the number one cause of human death in the developed world.
Traces of antibiotics were found in human skeletons from ancient times dating back to 350 – 550 CE. For example, traces of tetracycline have been found in human skeletal remains from ancient Sudanese Nubia dating back to 350–550 CE. The distribution of tetracycline in bones is only explicable after exposure to tetracycline-containing materials in the diet of these ancient people. Another example of ancient antibiotic exposure is from a histological study of samples taken from the femoral midshafts of the late Roman period skeletons from the Dakhleh Oasis in Egypt.
Tetracyclines are unique among antibiotics in that they are strong chelators and are incorporated into the hydroxyapatite mineral portion of bones as well as tooth enamel and thus provide permanent markers of metabolically active areas during tetracycline exposure.
Paul Ehrlich, a German physician, noted that certain chemical dyes coloured some bacterial cells but not others. He concluded that, according to this principle, it must be possible to create substances that can kill certain bacteria selectively without harming other cells.
In 1909, Paul Ehrlich discovered that a chemical compound called arsphenamine was an effective treatment for syphilis. This became the first modern antibiotic produced from a chemical compound. Ehrlich later referred to his discovery as ‘chemotherapy’ – the use of a chemical to treat a disease. The word ‘antibiotics’ was first used over 30 years later by the Ukrainian-American inventor and microbiologist Selman Waksman, who in his lifetime discovered over 20 antibiotics.
The development of anti-infective drugs and the underlying concept of chemotherapy is widely accredited to Paul Ehrlich, who developed the synthetic arsenic-based pro-drugs salvarsan (salvation arsenic) and neo-salvarsan circa 100 years ago to treat Treponema pallidum, the causative agent of syphilis.
Paul Ehrlich’s discovery of salvarsan, a synthetic drug represented one of the first systematic screens for drug discovery from various sources using a library of synthetic compounds. This sustained investment and continued search (till this day) for novel antimicrobial agents was inspired by Ehrlich’s work on dyes that specifically stained bacterial cells. Salvarsan was superseded by another set of drugs know as the sulfonamide prodrug Prontosil, discovered by Gerhard Domagk, a bacteriologist at Bayer who used the drug to save his daughter’s arm from amputation.
Following this, Alexander Fleming accidentally discovered penicillin in 1928. Upon returning from a holiday in Suffolk in 1928, he noticed that a fungus, Penicillium notatum, had contaminated a culture plate of Staphylococcus bacteria he had accidentally left uncovered. The fungus (P. notatum) had created bacteria-free zones wherever it grew on the plate. Fleming isolated and grew the mould in pure culture. He found that P. notatum proved extremely effective even at very low concentrations, preventing Staphylococcus aureus growth even when diluted 800 times, and was less toxic than the disinfectants used at the time.
This serendipitous discovery of penicillin by Alexander Fleming in 1928 started the golden age of natural product antibiotic discovery that peaked in the mid-1950s. Nonetheless, several efforts toward the development of antibiotics preceded Fleming’s discovery by at least several decades.
As scientists discovered that microbes could produce chemical substances that killed or inhibit other microbes, more and more antibiotics continued to be discovered over the years. Antibiosis between microbes was described well before the discovery of penicillin, including by Louis Pasteur, who proposed that microbes could secrete material to kill other bacteria or microbes.
Antibiosis is a phenomenon in which the growth of an organism is stopped (probably by another organism). Generally, antibiosis is the antagonism resulting from the toxicity of secondary metabolites produced by one microorganism for other microorganisms. Antibiosis is an antagonistic type of relationship between two organisms – in which one of the organisms suffers seriously, dies or prevented from growing further. Antibiosis is a biological interaction between two or more organisms that is detrimental to at least one of them.
Streptomycin, sulfa drugs, and penicillin were the first antibiotics to move from the laboratory into general clinical use. Subsequently, scientists have developed many new forms of these antibiotics and are currently looking for new types of antibiotics with targets different from those of the older antibiotics, some of which are no longer effective because of AMR. Soil bacteria and fungi are proved to be rich sources of antibiotics.
Source:
Aminov R.I (2010). A brief history of the antibiotic era: lessons learned and challenges for the future. Frontiers in Microbiology, 1-7.
Hutchings et al (2019). Antibiotics: past, present and future. Current Opinion in Microbiology, 51:72-80.
www.microbiologysociety.org/membership/membership-resources/outreach-resources/antibiotics-unearthed/antibiotics-and-antibiotic-resistance/the-history-of-antibiotics.html
Discover more from Microbiology Class
Subscribe to get the latest posts sent to your email.