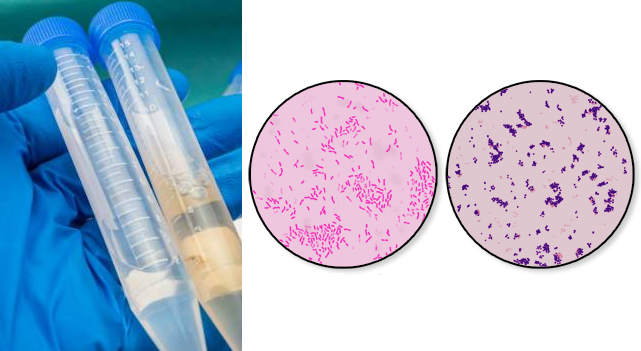
AIM: To detect the presence of pus cells and bacteria in cerebrospinal fluid (CSF) specimen.
MATERIAL/APPARATUS: CSF specimen, Gram staining reagents, microscope, glass slide, immersion oil, Bunsen burner.
METHOD/PROCEDURE FOR CSF GRAM STAINING
- Place a drop of the CSF specimen on a clean glass slide. Usually, a drop of the CSF sample is dropped on the glass slide from the syringe used in collecting the sample to avoid contamination.
- Make a thin smear of the CSF specimen.
- Allow to dry in a secure place to prevent it from dust.
- Heat-fix the smear by passing it thrice over the blue flame of a Bunsen burner.
- Perform the Gram staining technique.
- Allow the Gram stained slide to dry.
- Place a drop of immersion oil on the slide.
- View the slide under the microscope using the ×100/oil immersion objective lens.
REPORTING OF THE RESULT
Look for Gram negative intracellular diplococcic, Gram negative rods, Gram positive diplococci, and pus cells and report same. If the Gram smear contains bacteria and pus cells, inform the physician about it immediately. Culture the CSF specimen once the Gram smear proves positive for bacteria and pus cells. In cases of an emergency treatment when the patient is given antibiotic treatment before the CSF specimen is collected, it is usually possible and more difficult to detect bacteria in a Gram stained smear and to isolate bacteria from culture.
References
Basic laboratory procedures in clinical bacteriology. World Health Organization (WHO), 1991. Available from WHO publications, 1211 Geneva, 27-Switzerland.
Beers M.H., Porter R.S., Jones T.V., Kaplan J.L and Berkwits M (2006). The Merck Manual of Diagnosis and Therapy. Eighteenth edition. Merck & Co., Inc, USA.
Biosafety in Microbiological and Biomedical Laboratories. 5th edition. U.S Department of Health and Human Services. Public Health Service. Center for Disease Control and Prevention. National Institute of Health. HHS Publication No. (CDC) 21-1112.2009.
Cheesbrough M (2010). District Laboratory Practice in Tropical Countries. Part I. 2nd edition. Cambridge University Press, UK.
Cheesbrough M (2010). District Laboratory Practice in Tropical Countries. Part 2. 2nd edition. Cambridge University Press, UK.
Collins C.H, Lyne P.M, Grange J.M and Falkinham J.O (2004). Collins and Lyne’s Microbiological Methods. Eight edition. Arnold publishers, New York, USA.
Disinfection and Sterilization. (1993). Laboratory Biosafety Manual (2nd ed., pp. 60-70). Geneva: WHO.
Garcia L.S (2010). Clinical Microbiology Procedures Handbook. Third edition. American Society of Microbiology Press, USA.
Garcia L.S (2014). Clinical Laboratory Management. First edition. American Society of Microbiology Press, USA.
Fleming, D. O., Richardson, J. H., Tulis, J. I. and Vesley, D. (eds) (1995). Laboratory Safety: Principles and practice. Washington DC: ASM press.
Dubey, R. C. and Maheshwari, D. K. (2004). Practical Microbiology. S.Chand and Company LTD, New Delhi, India.
Gillespie S.H and Bamford K.B (2012). Medical Microbiology and Infection at a glance. 4th edition. Wiley-Blackwell Publishers, UK.
Discover more from #1 Microbiology Resource Hub
Subscribe to get the latest posts to your email.