Helping the physician select the best treatment at a much faster pace based on the right microbial identification and antimicrobial susceptibility test is crucial in any infectious disease management and patient total wellbeing. Clinical microbiology laboratories are the first lines of defense for the detection of antibiotic resistance from clinical pathogens and/or samples. And the current best approach for managing today’s problems of emerging infectious diseases and antimicrobial agent resistance to some commonly used and available antimicrobials is hinged on maintaining high-quality clinical microbiology laboratories in our hospitals – which will help provide good patient care outcomes through accurate and prompt identification of bacterial pathogens.
The clinical microbiology laboratories should also carry out accurate and prompt antimicrobial susceptibility test (AST) on clinical pathogens – as a panacea to guide physicians on the right choice of antimicrobial therapy for each. Services provided by the clinical microbiology laboratories should be carried out in a prompt, accurate and rapid manner since results from such sections of the hospital are critical to the optimal care of the patient. The VITEK 2 automated compact system has everything that the clinical microbiology laboratory needs for fast and accurate microbial identification, and for the performance of a fast and accurate antimicrobial susceptibility testing (AST).
VITEK 2 automated compact system is a fully automated high-throughput system that performs bacterial identification by biochemical analysis using colorimetry. This high-throughput technique for bacterial identification and AST mainly focuses on the clinical microbiology laboratory especially in the area of providing increased levels of automation and increased capacity for higher volume laboratories. The VITEK 2 automated compact system is suitable for both small and large laboratories since it does not occupy much space.
A colorimeter is a device used to test the concentration of a solution by measuring its absorbance of a specific wavelength of light while colorimetry is the technology that is used to quantify and describe physically the human perception of colour. Colorimetric equipment or equipments that work on the principle of colorimetry such as the VITEK 2 automated compact system is similar to that used in spectrophotometry (which is a method used to measure how much a chemical substance absorbs light by measuring the intensity of light as a beam of light passes through a sample solution).
The basic principle of the spectrophotometer is that each compound absorbs or transmits light over a certain range of wavelength. The advanced colorimetry of the VITEK 2 automated compact system provides high discrimination between microbial species – making microbial identification rapid and accurate. The VITEK 2 automated compact system for bacterial identification and AST also offers advanced colorimetric technology that allows for the identification of about 98 % of clinical pathogens from clinically-relevant samples.
Some of the notable features of the VITEK 2 compact automated system are elucidated as follows:
- The VITEK 2 automated compact system has a comprehensive updatable database of microbial strains; new generation of identification test cards; and it makes use of a Windows-based user-friendly software and computer system for easy processing of test results.
- The knowledge base of the VITEK 2 automated compact system is developed from about 100,000 references of microbial strains.
- The system described over 2000 microbial phenotypes.
- It has over 20,000 minimum inhibitory concentration (MIC) distributions for a variety of antimicrobial agents or antibiotics.
- The VITEK 2 automated compact system can detect over 100 resistance mechanisms in microbial pathogens.
- It provides a resulting range of five to seven MIC doubling dilutions per antibiotic on the average.
- It has an extended MIC range to enable low-level resistance detection.
- It deduces antibiotic results to meet formulary requirements in terms of prescribing the correct antimicrobial therapy.
- It is fast, rapid and accurate; and it checks every result every time.
The VITEK 2 automated compact system provides accurate “fingerprint” recognition of bacterial resistance mechanisms and phenotypes as well as prompt and accurate AST results. The components of the VITEK 2 compact automated system for microbial identification and AST include: the reader/incubator, waste collection bin, cassette load/unload station, fill door, front user access door, top user access door, filler station, loading/load door and waste collection door (Figure 1).
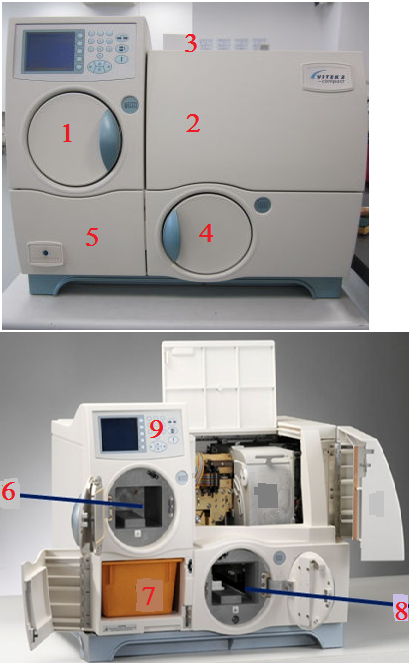
BENEFITS OF THE VITEK 2 AUTOMATED COMPACT SYSTEM
- It enhances workflow in clinical microbiology laboratory because of the short time it takes in processing clinical samples and/or isolates.
- It enables rapid reporting of laboratory test results and reduces hands-on time which usually causes delay in cultural techniques of microbial identification and AST.
- It reduces time to microbial (yeast and bacteria) identification.
- It reduces time for obtaining antibiotic susceptibility testing results.
- It offers an extensive identification and susceptibility menu.
- It reduces waste with a miniaturized card-format that measures 10 cm x 6 cm x 0.5 cm and weighs only 16 grams (Figure 2).
- The VITEK 2 automated compact system is compartmentalized; and thus it meet the needs of any size laboratory including small and large laboratories.
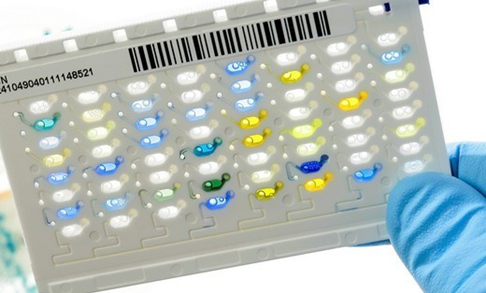
References
Arora D.R (2004). Quality assurance in microbiology. Indian J Med Microbiol, 22:81-86.
Ashutosh Kar (2008). Pharmaceutical Microbiology, 1st edition. New Age International Publishers: New Delhi, India.
Barenfanger J, Drakel C and Kacich (1999). Clinical and Financial Benefits of Rapid Bacterial Identification and Antimicrobial Susceptibility Testing. Journal of Clinical Microbiology, 37(5):1415-1418.
Denyer S.P., Hodges N.A and Gorman S.P (2004). Pharmaceutical Microbiology. 7th ed. Blackwell Publishing Company, USA.
Doern G, Brueggemannn A.B, Perla R, Daly D, Halkias D, Jones R.N, Saubolle M.A (1997). Multicenter laboratory evaluation of the bioMerieux Vitek antimicrobial susceptibility testing system with 11 antimicrobial agents versus members of the family Enterobacteriaceae and Pseudomonas aeruginosa. J Clin Microbiol, 35:2115–2119.
Doern G, Vautour R, Gaudet M, Levy B (1994). Clinical impact of rapid in vitro susceptibility testing and bacterial identification. J Clin Microbiol, 32:1757–1762.
Doern G.V (1995). Susceptibility tests of fastidious bacteria. Manual of Clinical Microbiology, 6th edition, Murray P.R, Baron E.J, Pfaller M.A, Tenover F.C, Yolken R, American Society for Microbiology, Washington DC, Pp. 1342-1349.
Funke G, Monnet D, deBernardis C, von Graevenitz A, Freney J (1998). Evaluation of the VITEK 2 system for rapid identification of medically relevant Gram-negative rods. J Clin Microbiol, 36:1948–1952.
Garcia L.S (2010). Clinical Microbiology Procedures Handbook. Third edition. American Society of Microbiology Press, USA.
Hart C.A (1998). Antibiotic Resistance: an increasing problem? BMJ, 316:1255-1256.
Livermore D.M, Winstanley T.B, Shannon K.P (2001). Interpretative reading: recognizing the unusual and inferring resistance mechanisms from resistance phenotypes.
J Antimicrob Chemother, 48 Suppl 1:87-102.
Madigan M.T., Martinko J.M., Dunlap P.V and Clark D.P (2009). Brock Biology of Microorganisms, 12th edition. Pearson Benjamin Cummings Inc, USA.
Mahon C. R, Lehman D.C and Manuselis G (2011). Textbook of Diagnostic Microbiology. Fourth edition. Saunders Publishers, USA.
National Committee for Clinical Laboratory Standards. Performance Standards for antimicrobial susceptibility testing. 8th Informational Supplement. M100 S12. National Committee for Clinical Laboratory Standards, 2002. Villanova, Pa.
Washington J.A (1993). Rapid antimicrobial susceptibility testing: technical and clinical considerations. Clin Microbiol Newsl, 15:153–155.
Discover more from #1 Microbiology Resource Hub
Subscribe to get the latest posts to your email.


