PRESUMPTIVE TEST
The presumptive test provides a preliminary estimate of bacterial density present in a test sample based on enrichment in minimally restrictive tube (broth) culture media. It is the first step of the MPN procedure for feacal coliform testing carried out on public health samples including water, milk and food. The original test sample is thoroughly mixed and divided into pre-determined sub-volumes with or without dilution. This test looks for the presence of feacal coliforms in the test (water) sample by inoculating lactose broths with the water sample. It is noteworthy that the results of the presumptive tests are never used without further analysis such as conducting the confirmed and completed tests.
In carrying out the presumptive test, the samples are first inoculated into lactose broth contained a Durham tube. The tubes are incubated at the appropriate environmental conditions and checked for the production of gas and acid. In a positive culture, growth will occur with the production of both acid and gas. Those Durham’s tubes that show presence or production of acid and gas are scored as positive (+) tubes and those with no acid/gas production are scored as negative(–) tubes. Usually, three or five sets of lactose broths are inoculated with varying dilutions of the test sample.
The first set of 3 or 5 Durham’s tubes is inoculated with 10 ml of sample while the second set of tubes is inoculated with 1 ml of the test sample (Figure 1). A third set of tubes can be included in the experiment. This third set of tube is inoculated with 0.1 ml of the test sample. Each set of the Durham’s tubes (3 or 5 sets of tubes) receives a measured amount of the test sample; and the amount of sample added to the tubes is usually dependent in part by the expected concentration of coliform bacteria in the test sample.
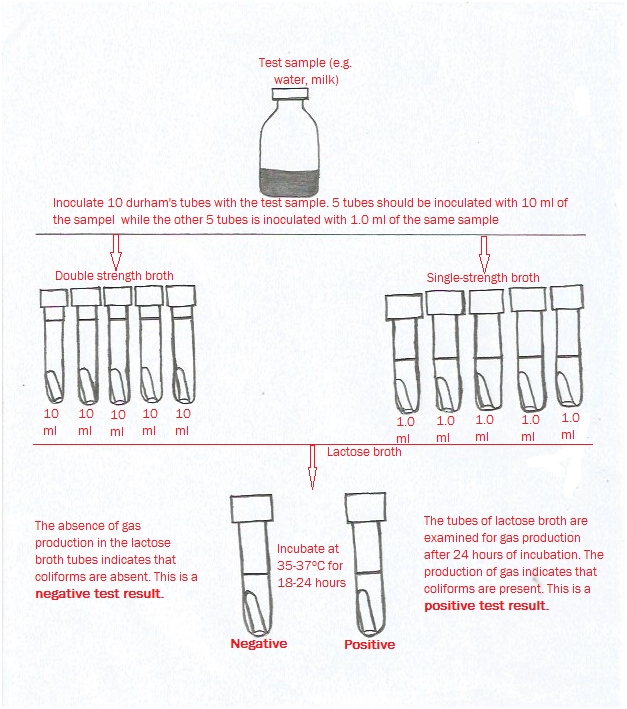
It is vital that the second set of the tubes receives an amount of the test sample that is 10-fold less than the first sets of tubes; and the third sets of tubes also receives an amount of the tests samples that is 100-fold less than the previous (second sets of tubes) tubes. This implies that each set of the tubes receives an amount of the test sample that is 10-fold lesser than the previous sets of tubes. A positive presumptive test is a very good indication of the presence of feacal coliform or coliforms in a test sample – since only few other types of bacteria can ferment lactose and yield acid and gas the same way coliforms do. The presumptive tests are generally designed to grow the target bacteria.
CONFIRMED TEST
The confirmed test is the second step of the MPN procedure which detects the presence of feacal coliforms in the test sample. In the confirming test procedure for feacal coliform bacteria, the positive presumptive cultures are transferred to selective culture media including Eosin Methylene Blue (EMB) agar and Brilliant Green Lactose Bile Broth (BGLBB) medium specific for feacal coliform bacteria. And any presumptive tube transfer which shows gas production after 24 hours of incubation at 35°C confirms the presence of feacal coliform bacteria in that tube and is recorded as a positive confirmed tube.
Some non-coliform bacteria can yield a false positive result in the presumptive test. Thus, all positive lactose broth cultures are subjected to the confirmed test. In carrying out the confirmed test, a loopful of each broth culture is streaked onto Eosin Methylene Blue (EMB) agar and also inoculated into Brilliant Green Lactose Bile Broth (BGLBB) medium (Figure 2). EMB is a differential medium because eosin and methylene blue combine to yield a distinctive coloration pattern for coliform bacteria.
The presence of bile and brilliant green in BGLBB medium make this medium selective for only Gram-negative bacteria including the coliforms. A confirmed test is positive when colonies with a green metallic sheen form on EMB and gas production occurs in BGLBB. The media used in the confirmed tests are designed to validate the growth of target bacteria in the presumptive test. Confirmed test conditions are usually more stringent than presumptive test conditions.
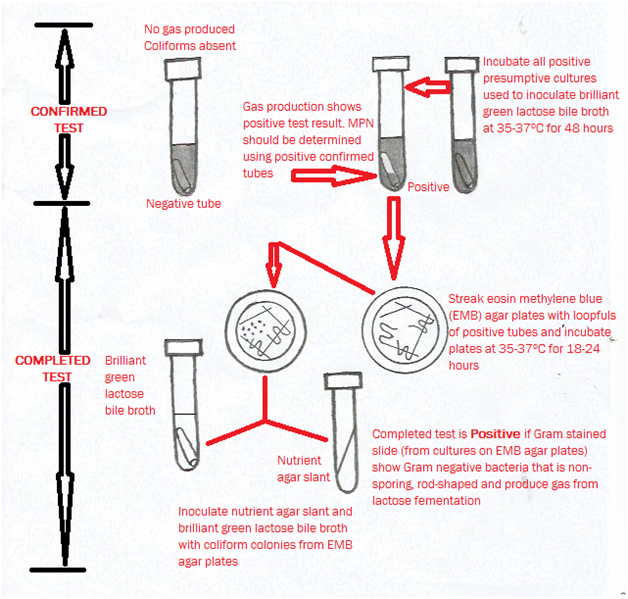
COMPLETED TEST
The completed test is the third and last stage of the MPN procedure. In the completed test, cells from an isolated colony on EMB agar are transferred to an agar slant and again transferred to lactose broth. If acid and gas are again observed, and Gram-negative rods are found in the slant culture, then the identification of coliform is considered positive. Microbiological and/or biochemical testing’s including but not limited to Gram staining, microscopy and sugar fermentation tests are usually carried out at this stage in order to identify the contaminating coliform bacteria in the test sample.
ADVANTAGES OF MPN METHOD
- MPN method is relatively simple to perform.
- It is sensitive and can be used to analyze samples with relative fewer numbers of bacteria.
- The MPN technique can be used to count or estimate a specific type of bacteria in the presence of others – since it make makes use of differential media.
- MPN method can use large sample volumes for bacteria estimation.
DISADVANTAGES OF MPN METHOD
- MPN method is time consuming and labor intensive. A lot of space, glassware, test tubes and culture media (broth) is usually required to perform an MPN analysis
- Requires large volumes of glassware
- MPN method does not give the real value of bacterial count. It only gives an estimate of the number of bacteria present in the sample.
- MPN technique does not give isolated colonies as is applicable in plate count technique.
References
Abrahams P.W (2006). Soil, geography and human disease: a critical review of the importance of medical cartography. Progress in Physical Geography, 30:490-512.
Ahring B.K, Angelidaki I and Johansen K (1992). Anaerobic treatment of manure together with industrial waste. Water Sci. Technol, 30, 241–249.
Andersson L and Rydberg L (1988). Trends in nutrient and oxygen conditions within the Kattegat: effects on local nutrient supply. Estuar. Coast. Shelf Sci, 26:559–579.
Ballantyne A.P, Alden C.B, Miller J.B, Tans P.P and White J.W.C (2012). Increase in observed net carbon dioxide uptake by land and oceans during the past 50 years. Nature, 488: 70-72.
Baumgardner D.J (2012). Soil-related bacterial and fungal infections. J Am Board Fam Med, 25:734-744.
Bennett E.M, Carpenter S.R and Caraco N.F (2001). Human impact on erodable phosphorus and eutrophication: a global perspective. BioScience, 51:227–234.
Bunting B.T. and Lundberg J (1995). The humus profile-concept, class and reality. Geoderma, 40:17–36.
Chang S.T (2006). The world mushroom industry: trends and technological development. International J. Medicinal Mushrooms, 8:297-314.
Jee C and Shagufta (2007). Environmental Biotechnology. APH Publishing Corporation, Darya Ganj, New Delhi, India.
Maier R.M, Pepper I.L. and Gerba C.P (2000). Environmental Microbiology. Academic Press, San Diego.
Miguel A, Manuel F, Francisco J.P and Antonio B (2006). Environmental biocatalysis: from remediation with enzymes to novel green processes. TRENDS in Biotechnology, 24(6):1-7.
Mishra B.B, Nanda D.R and Dave S.R (2009). Environmental Microbiology. First edition. APH Publishing Corporation, Ansari Road, Darya Ganj, New Delhi, India.
Paul E.A (2007). Soil Microbiology, ecology and biochemistry. 3rd edition. Oxford: Elsevier Publications, New York.
Pelczar M.J Jr, Chan E.C.S, Krieg N.R (1993). Microbiology: Concepts and Applications. McGraw-Hill, USA.
Pelczar M.J., Chan E.C.S. and Krieg N.R. (2003). Microbiology of Soil. Microbiology, 5th Edition. Tata McGraw-Hill Publishing Company Limited, New Delhi, India.
Pepper I.L and Gerba C.P (2005). Environmental Microbiology: A Laboratory Manual. Second Edition. Elsevier Academic Press, New York, USA.
Roberto P. Anitori (2012). Extremophiles: Microbiology and Biotechnology. First edition. Caister Academic Press, Norfolk, England.
Salyers A.A and Whitt D.D (2001). Microbiology: diversity, disease, and the environment. Fitzgerald Science Press Inc. Maryland, USA.
Sawyer C.N, McCarty P.L and Parkin G.F (2003). Chemistry for Environmental Engineering and Science (5th ed.). McGraw-Hill Publishers, New York, USA.
Ulrich A and Becker R (2006). Soil parent material is a key determinant of the bacterial community structure in arable soils. FEMS Microbiol Ecol, 56(3):430–443.
Discover more from #1 Microbiology Resource Hub
Subscribe to get the latest posts to your email.


