The estimation of the number of microorganisms including bacteria and fungi present in public health sample including water, food, beverage, or other products is one of the major tasks of microbiologists especially those working in water treatment plants, food industries and in other government public health establishments. After isolation and estimating the number of microorganisms present in the samples, the microbiologists also goes further to determine which types of microorganisms are present in the analyzed sample in order to assess the quality of the product.
Several techniques are used for the enumeration of microorganisms in any given sample. While some of these techniques may be molecularly-inclined (such as the use of polymerase chain reaction, PCR techniques or DNA probes), others are traditionally inclined (such as the most probable number method) and have been used by microbiologists for several decades in the estimation of the numbers of microbes present in a sample.
Some of the notable techniques used by microbiologists in deciphering the organisms present in a given sample include: direct microscopic count, electronic particle count, use of DNA probes, most probable number method, dilution/spread plating technique, dilution/pour plating technique, and the membrane filtration technique – which uses membrane filters of various sizes for microbial enumeration.
Most probable number (MPN) is a quantitative analysis that is used for estimating the number of viable microorganisms (usually bacteria) suspended in a given liquid sample including food sample, water sample and soil sample. MPN is a statistical estimate of viable cell numbers based on the theory of probability. The main goal of doing MPN analysis is to successively dilute a given sample in order to determine the point at which subsequent dilutions receive no cells.
It is generally used for the bacteriological analysis of public health samples such as water and food. MPN is useful for those samples including milk and water whose bacteria concentration may be low (<100/g), and thus difficult to estimate by other conventional laboratory techniques. Estimation of the numbers of bacteria is often made using dilution series, for example when examining food or water samples for the presence of coliforms.
Coliforms or coliform bacteria are bacteria that serve as indicators of feacal contamination of samples including water, milk and food. Coliform bacteria are not themselves pathogenic but they are occupants of the digestive systems of humans and animals; and their presence in the human digestive system makes them to be abundant in human feacal matter.
Coliform bacteria are Gram negative, aerobic or facultative anaerobes and non-sporing rod-like bacteria that are notable for their ability to ferment sugars such as lactose with the resultant production of gas and acid. This biochemical characteristic of the coliforms amongst others makes their identification from other bacteria somewhat easier.
Feacal coliform bacteria are thus non‑disease causing microorganisms which are found in the intestinal tract of all warm-blooded animals. The number of feacal coliform bacteria present in a sample such as water is a good indicator of the amount of pollution present in the water. However, it is noteworthy that the presence of coliforms in a sample does not necessarily suggest that human pathogens are present in the sample.
Rather, the presence of coliform bacteria in a given public health sample merely suggest that feacal contamination of the sample has occurred, and that potential pathogens may be present in the sample. MPN is one of the methods of counting viable bacteria in a sample.
The standard plate count – in which bacteria in a sample are counted based on colony formation on solid culture media is another technique employed in counting bacteria in a given sample. While the MPN technique is usually used for samples that have a relatively low number of bacteria (in which case the microbial cells will not be detected if the samples were cultured on agar plates), the standard plate counting technique is used for samples that have a relatively large number of bacteria. MPN which can also be called the multiple tube method is only used to estimate or enumerate viable organisms in a sample especially those samples suspected to contain relatively low numbers of bacteria as aforementioned.
Direct plating methods (plate counts) gives a direct count of viable (living) microorganisms present in a sample, and this is usually expressed in colony forming units (CFU) – since no single culture medium will support the growth of all different bacteria at a particular time. And thus, the bacteria is counted and expressed as CFU since we can only count those bacteria that do grow to form a visible colony on the culture medium.
MPN analysis only gives an estimate of the viable organisms present in the sample; and as aforementioned, MPN values are particularly useful when low concentrations of the microorganisms (<100/g) are encountered in the sample (food, milk, soil, water) – where particulate matter of the matrix (or environment the sample is ensuing from) may interfere with obtaining accurate colony counts of the bacterial population present in the test sample. MPN is usually a cumbersome type of bacteriological analysis to conduct in the microbiology laboratory; and this is usually due in part to the space it occupies in the laboratory – and owing to the fact that multiple tubes are used in carrying out the test. A lot of space, glassware, test tubes and culture media (broth) is usually required to perform an MPN analysis.
The tedious nature of carrying out MPN in the laboratory makes it prone to some erroneous results or errors especially errors due to limited surface area for colony formation of bacteria in test tubes used. Nevertheless, the MPN method still remains a veritable tool for estimating the population density of viable microorganisms in public health test samples including water, milk and food. MPN analysis is a statistical bacteriological test method that is based upon the probability theory. The principle of the MPN test is based on the application of the theory of probability to the numbers of observed positive growth responses to a standard dilution series of sample inoculum placed in a set number of culture media (broth) tubes.
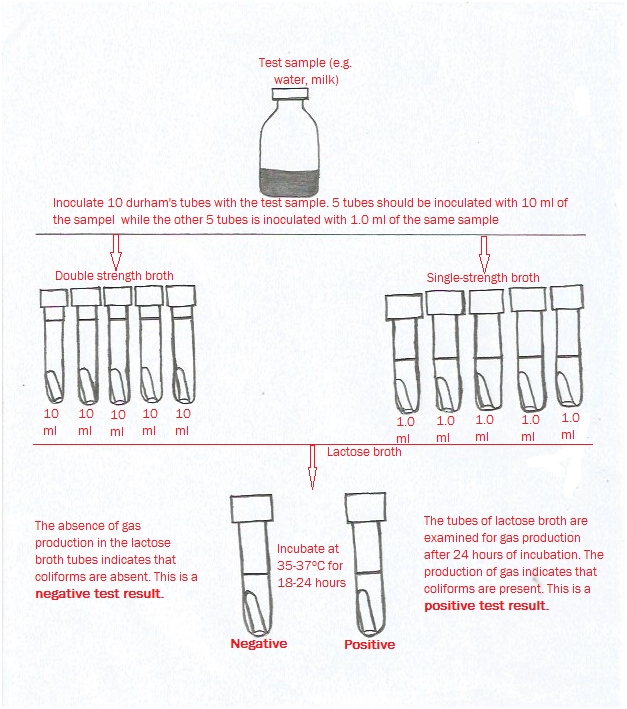
The production of visible turbidity in the test tubes or gas production by the microorganism is usually used to ascertain positive growth response of the organism after incubation of the test tubes containing the sample and the broth at the appropriate growth/environmental conditions. Fermentation tubes and broth tubes are usually the two types of culture media protocols used for carrying out the MPN analysis. While microbial growth is determined in the fermentation tubes as “gas production”; a visible turbidity is used to estimate or detect microbial growth in the broth tubes.
In carrying out MPN analysis, the number of sample dilutions to be prepared is usually dependent on the expected microbial population contained in the test sample; and samples for MPN analysis are usually diluted in such a way that higher dilutions of the test sample will result in fewer positive culture tubes in the series. MPN is based on the probability of finding bacterial growth after culture and incubation of successive dilutions of the test sample in a liquid (broth) medium. It is assumed that bacteria present in a sample are normally distributed in liquid media in a random manner. This implies that repeated samples of the same size from one source are expected to contain the same number of microorganisms on average even though some of the test samples will obviously contain a few more bacteria and the others a few less bacteria.
The average number of bacteria estimated from the tubes and/or cultured and incubated samples is the most probable number (MPN). If the number of microorganisms is large, the differences between samples will be small; and all the individual results will be nearer to the average. But if the number of microorganisms is small the difference will be relatively larger. For example, if a liquid sample (e.g. liquid milk or water) contains 100 microorganisms per 100 ml, it then implies that 10 ml samples will contain on the average 10 microorganisms each.
Though some of the samples will contain more than 10 microorganisms while some will contain less than 10 microorganisms, it is unlikely to have a sample that does not contain any microorganism. It is expected that every of these individual samples will show microbial growth when inoculated into a suitable culture medium (broth) and incubated at the appropriate temperature. Test samples are serially diluted to the point of extinction (i.e. to a point where there are no more viable microorganisms present in the sample).
To detect the end point, multiple serial dilutions of the test sample are inoculated into a suitable growth medium (usually a broth), and the development of some recognizable growth characteristic, such as gas production, acid production or turbidity, is used to indicate microbial growth (the presence of at least one viable microorganism in the diluted sample). The pattern of positive tests (growth) in the replicates and statistical probability tables are used to determine the concentration (most probable number) of bacteria in the original test sample.
Statistical MPN tables are available for replicates of 3, 5, and 10 Durham’s tubes of each dilution. The more replicate tubes used, the greater the precision of the estimate of the size of the bacterial population. The MPN method is an alternative to plate counts or membrane techniques, especially for samples with higher turbidity. The number of bacteria per 100 ml of sample is estimated by the use of probability tables.
The basic concept for the MPN method is to dilute the sample to be analyzed to such a degree that the microorganisms (inocula) in each of the Durham’s tubes will sometimes (but not always) contain viable organisms. Thus by replicates, and dilution series, this will result in a fairly accurate estimate of the most probable number of cells in the analyzed sample.
MPN technique has three test stages which includes (1) The Presumptive Test, (2) The Confirmed Test and (3) The Completed Test. Before the Durham’s tubes are inoculated with the test bacterium, the chance is at least 95 percent that the confidence interval associated with the eventual result will enclose the actual concentration of the sample; and this signifies the meaning of the 95 percent confidence intervals in the MPN Table.
ADVANTAGES OF MPN METHOD
- MPN method is relatively simple to perform.
- It is sensitive and can be used to analyze samples with relative fewer numbers of bacteria.
- The MPN technique can be used to count or estimate a specific type of bacteria in the presence of others – since it make makes use of differential media.
- MPN method can use large sample volumes for bacteria estimation.
DISADVANTAGES OF MPN METHOD
- MPN method is time consuming and labor intensive. A lot of space, glassware, test tubes and culture media (broth) is usually required to perform an MPN analysis
- Requires large volumes of glassware
- MPN method does not give the real value of bacterial count. It only gives an estimate of the number of bacteria present in the sample.
- MPN technique does not give isolated colonies as is applicable in plate count technique.
References
Abrahams P.W (2006). Soil, geography and human disease: a critical review of the importance of medical cartography. Progress in Physical Geography, 30:490-512.
Ahring B.K, Angelidaki I and Johansen K (1992). Anaerobic treatment of manure together with industrial waste. Water Sci. Technol, 30, 241–249.
Andersson L and Rydberg L (1988). Trends in nutrient and oxygen conditions within the Kattegat: effects on local nutrient supply. Estuar. Coast. Shelf Sci, 26:559–579.
Ballantyne A.P, Alden C.B, Miller J.B, Tans P.P and White J.W.C (2012). Increase in observed net carbon dioxide uptake by land and oceans during the past 50 years. Nature, 488: 70-72.
Baumgardner D.J (2012). Soil-related bacterial and fungal infections. J Am Board Fam Med, 25:734-744.
Bennett E.M, Carpenter S.R and Caraco N.F (2001). Human impact on erodable phosphorus and eutrophication: a global perspective. BioScience, 51:227–234.
Bunting B.T. and Lundberg J (1995). The humus profile-concept, class and reality. Geoderma, 40:17–36.
Chang S.T (2006). The world mushroom industry: trends and technological development. International J. Medicinal Mushrooms, 8:297-314.
Jee C and Shagufta (2007). Environmental Biotechnology. APH Publishing Corporation, Darya Ganj, New Delhi, India.
Maier R.M, Pepper I.L. and Gerba C.P (2000). Environmental Microbiology. Academic Press, San Diego.
Miguel A, Manuel F, Francisco J.P and Antonio B (2006). Environmental biocatalysis: from remediation with enzymes to novel green processes. TRENDS in Biotechnology, 24(6):1-7.
Mishra B.B, Nanda D.R and Dave S.R (2009). Environmental Microbiology. First edition. APH Publishing Corporation, Ansari Road, Darya Ganj, New Delhi, India.
Paul E.A (2007). Soil Microbiology, ecology and biochemistry. 3rd edition. Oxford: Elsevier Publications, New York.
Pelczar M.J Jr, Chan E.C.S, Krieg N.R (1993). Microbiology: Concepts and Applications. McGraw-Hill, USA.
Pelczar M.J., Chan E.C.S. and Krieg N.R. (2003). Microbiology of Soil. Microbiology, 5th Edition. Tata McGraw-Hill Publishing Company Limited, New Delhi, India.
Pepper I.L and Gerba C.P (2005). Environmental Microbiology: A Laboratory Manual. Second Edition. Elsevier Academic Press, New York, USA.
Roberto P. Anitori (2012). Extremophiles: Microbiology and Biotechnology. First edition. Caister Academic Press, Norfolk, England.
Salyers A.A and Whitt D.D (2001). Microbiology: diversity, disease, and the environment. Fitzgerald Science Press Inc. Maryland, USA.
Sawyer C.N, McCarty P.L and Parkin G.F (2003). Chemistry for Environmental Engineering and Science (5th ed.). McGraw-Hill Publishers, New York, USA.
Ulrich A and Becker R (2006). Soil parent material is a key determinant of the bacterial community structure in arable soils. FEMS Microbiol Ecol, 56(3):430–443.
Discover more from #1 Microbiology Resource Hub
Subscribe to get the latest posts to your email.