Purines are heterocyclic aromatic organic compound that consist of a pyrimidine ring fused to an imidazole ring. Purines are the larger of the two types of bases found in DNA. Adenine (A) and guanine (G) are purines. Adenine is always paired with thymine, and guanine is always paired with cytosine.
Purines unlike pyrimidines (which have only one ring structure) have a double ring structure. Only two purines and three pyrimidines occur widely in nucleic acids. Purines form hydrogen bonds with their complementary pyrimidines during the synthesis of nucleic acid molecules (i.e. DNA and RNA). In DNA, the purines adenine (A) and guanine (G) pair up with the pyrimidines thymine (T) and cytosine (C), respectively while in RNA, the complement of adenine (A) is uracil (U) instead of thymine (as is applicable in DNA).
Purines are natural substances found in all of the body’s cells, and in virtually all foods. They provide part of the chemical structure of our genes and the genes of plants and animals. Purines are found in high concentration in meat and meat products, especially internal organs such as liver and kidney; and they are found in some plant materials in low concentration.
PYRIMIDINES
Pyrimidines are aromatic heterocyclic organic molecules or compounds found in living organisms. They are one of two biologically important families of nitrogen-containing molecules called nitrogenous bases.
The other family of nitrogenous bases is the purines as aforementioned. Pyrimidines can be identified by their structure: six atoms in the shape of a ring. Pyrimidines like the purines are amongst the building blocks of nitrogenous bases – which are integral parts of the molecular structure so most living systems.
The pyrimidines have only two nitrogen atoms in their ring; and the compound is a six-membered heterocyclic molecule (Table 1).
Table 1. Summary of the structures of the components of a nucleotide molecule
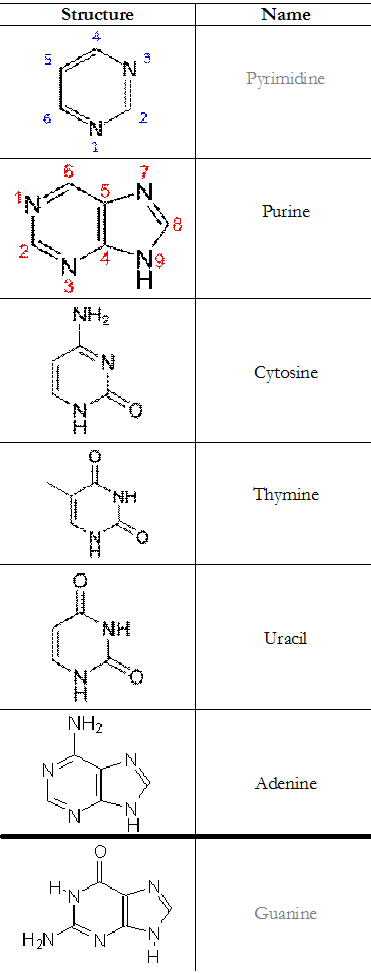
The nitrogen atoms of the pyrimidines are usually located at positions one (1) and three (3) as shown in Table 1. Chemically, pyrimidines are known as diazine molecules (because of their six-membered structure); and they a found in a variety of compounds and/or molecules especially the nucleic acid molecules – which harbour the genetic materials of living organisms.
Pyrimidines occur in various compounds found in nature and also in some synthetic compounds including but not limited to nucleotides as aforementioned, HIV/AIDS drugs (e.g. zidovudine), vitaminB1 (thiamine), some antibiotics (e.g. trimethoprim), alkaloids from plants, and barbiturates.
Most pharmacological agents and/or drugs used for the treatment of infectious diseases in man are usually based on the pyrimidine ring. This simply implies that these agents are synthetically manufactured based on the structure of the pyrimidine molecule.
Pyrimidines serve as the building blocks for DNA when paired with the purines; and their most important function in living organisms is in the synthesis of DNA molecules. Cytosine (C), thymine (T) and uracil (U) are the three main pyrimidine derivatives.
Adenosine triphosphate is a typical example of a nucleotide molecule (ATP) because it contains all the components that make up a nucleotide molecule as aforementioned (Figure 1).
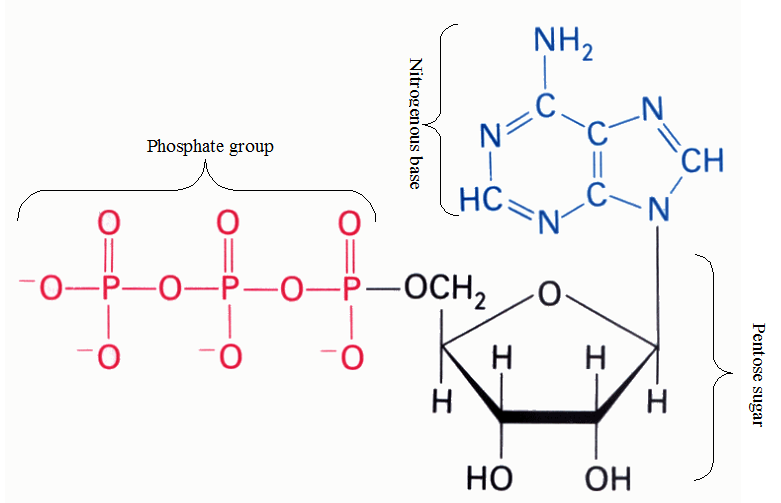
Figure 1. Structure of adenosine triphosphate (ATP).
ATP is a typical example of a nucleotide. A nucleotide comprises of three different molecules joined together; and these molecules are nitrogenous base, pentose sugar, and a phosphate group. ATP is the energy currency of the cell; and it is broken down to adenosine diphosphate (ADP) and inorganic phosphate (Pi) molecules in the cells during cellular and metabolic reactions that occur in the cell of living systems.
ATP releases free energy required by the cell for a variety of cellular functions such as energy required for the building of new molecules in the cell. ATPase is the enzyme that catalyzes the breakdown of ATP to ADP and Pi. Energy is mainly stored in the cells of both eukaryotic and prokaryotic organisms as ATP; and ATP is usually the energy reserve of the cell.
References
Alberts B, Bray D, Johnson A, Lewis J, Raff M, Roberts K andWalter P (1998). Essential Cell Biology: An Introduction to the Molecular Biology of the Cell. Third edition. Garland Publishing Inc., New York.
Dale J (2003). Molecular genetics of bacteria. Jeremy W. Dale and Simon Park (4th eds.). John Wiley & Sons Ltd, West Sussex, UK. Pp. 312-313.
Dale J (2003). Molecular genetics of bacteria. Jeremy W. Dale and Simon Park (4th eds.). John Wiley & Sons Ltd, West Sussex, UK. Pp.
Glick B.R and Pasternak J.J (2003). Molecular Biotechnology: Principles and Applications of Recombinant DNA. ASM Press, Washington DC, USA.
Madigan M.T., Martinko J.M., Dunlap P.V and Clark D.P (2009). Brock Biology of Microorganisms, 12th edition. Pearson Benjamin Cummings Inc, USA.
Tamarin Robert H (2002). Principles of Genetics. Seventh edition. Tata McGraw-Hill Publishing Co Ltd, Delhi.
Thieman W.J, Palladamo M.A and Thieman W (2003). Introduction to Biotechnology. Benjamin Cummings, San Francisco, CA.
Discover more from #1 Microbiology Resource Hub
Subscribe to get the latest posts to your email.