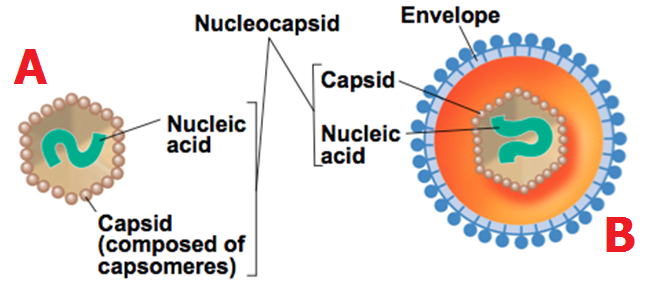
Prions are sub-viral infectious entities that consist mainly of proteins. They are unique infectious particles that lack nucleic acids inclusive of DNA and RNA. Prions are infectious proteins that generally lack DNA and RNA; and they cause series of diseases in man and animals. They differ from the normal virion in so many ways. Prions are non-immunogenic while viruses are immunogenic.
The immune system of mammals is incapacitated to mount an immune response to the invasion of host cells by pathogenic prion proteins. Viruses possess an RNA or DNA genome that is completely devoid in prions. Prions are composed mainly of an abnormal, pathogenic isoform that is the only identifiable macromolecule in purified preparations of prions.
These identifiable macromolecules of prions are generally known as prion protein scrapie (PrPSc), and the PrPSc are the pathogenic forms of the prion protein (PrP). The native cellular isoform of the prion protein which are normally found in mammalian cells are known as prion protein cellular (PrPC). Mammalian prions reproduce by recruiting the normal, cellular isoform of the prion protein (PrPC) and stimulating its conversion into the disease-causing isoform or pathogenic forms of the prion proteins (PrPSc).
Though they lack nucleic acids (which are unique in carrying genetic information for disease initiation in a particular host), the prions cause diseases in animals and mammals as aforementioned. Kuru disease (that occur in cannibalistic mammals), Creutzfeldt- Jakob disease (CJD) that occur in humans, scrapie (that occurs in goat and sheep), bovine spongiform encephalopathy (BSE) that occur in cattle and chronic wasting disease (that occur in deer and elk) amongst others are typical examples of prion diseases in animals and mammals (Table 1).
Transmissible spongiform encephalopathy (TSE) is the fatal prion disease of the nervous system that is characterized by the degeneration of the spongiform of the brain in animals. Animal prion diseases are collectively known as transmissible spongiform encephalopathies (TSEs). Since prions lack nucleic acid molecules, how then do they replicate in vivo to initiate a disease process or produce their own progeny from parental cells? Prion molecules as aforementioned are mainly made up of PrP; and the host cell usually contains a gene that encodes the native form of the prion proteins (i.e. PrPC).This gene in the host cell that encodes for PrPC is known as the human PrP gene (Prnp); and the gene is located in chromosome number 20 in humans and chromosome number 2 in mice.
Table 1. Summary of some prion diseases in man and animals
| Prion disease | Natural host |
| Kuru | Humans |
| Scrapie | Sheep and goat |
| Creutzfeldt-Jakob disease (CJD) | Humans |
| Chronic wasting disease | Elk and deer |
| Fatal familial insomnia (FFI) | Humans |
| Bovine spongiform encephalopathy (BSE) | Cattles |
| Fatal sporadic insomnia (FSI) | Humans |
| Feline spongiform encephalopathy (FSE) | Cats |
| Gerstmann-Straussler-Scheinker syndrome (GSSS) | Humans |
The pathogenic form of prion proteins (i.e. PrPSc) is identical to the cellular isoform of the prion protein (PrPC), which is non-pathogenic in nature. This genetic similarity between the epitope of PrPC and PrPSc is responsible for the non-immunogenicity of prions, and the reason why host cells fails to mount an immune response to prion invasion the same it mounts an attack against viral agents that enter the body.
The invasion of a host cell by PrPSc (pathogenic prion protein) stimulates the genetic conversion of PrPC (the normal and non-infectious prion protein)to PrPSc; and this is particularly applicable in host cells that are expressing PrPC. PrPC and PrPSc are the two known conformations in which prion proteins exist. It is however noteworthy that the replication of prions in host cells is generally facilitated when the sequences of the PrPC and PrPSc are identical.
Thus the presence of PrPC is vital for the development of prion diseases in mammals and animals; and this has been confirmed in knock-out mice (i.e. mice whose Prnp gene have been genetically modified) which are unable to form the PrPC proteins and the prion disease after several challenges with PrPSc, the pathogenic prion proteins. A pathogenic prion protein (PrPSc) replicates itself in host cells be converting healthy prion proteins (PrPC) to the pathogenic isoforms (i.e. PrPSc). PrPCisnormally produced by cells of the neurons in mammals and animals; and a misfolding of the PrPC during its synthesis in the neuron can lead to the conversion of PrPC to PrPSc which is insoluble and resistant to proteases (protein degrading enzymes).
The formation of PrPSc leads to the development of aggregates of the pathogenic prion proteins, and this phenomenon causes a damage of the neural tissues, brain tissues and other neurological symptoms that causes prion diseases to ensue. Profound neurological dysfunction in animal and mammalian hosts infected by pathogenic prion proteins is usually fatal in nature. Prion diseases are generally initiated in host cells by a molecular mechanism that causes pathogenic prion proteins to arise spontaneously; and infectious prion proteins are infectious and can be transferred from one infectious host to another susceptible host.
Prions have also been discovered to infect fungi especially yeast cells aside animals and mammalian cells that they are mainly known to infect. There is currently no therapeutic option for the treatment of prion diseases even though several compounds have been tested and suggested for use in the management of neurodegenerative diseases caused by pathogenic prion proteins.
References
Acheson N.H (2011). Fundamentals of Molecular Virology. Second edition. John Wiley and Sons Limited, West Sussex, United Kingdom.
Alan J. Cann (2005). Principles of Molecular Virology. 4th edition. Elsevier Academic Press, Burlington, MA, USA.
Alberts B, Bray D, Johnson A, Lewis J, Raff M, Roberts K and Walter P (1998). Essential Cell Biology: An Introduction to the Molecular Biology of the Cell. Third edition. Garland Publishing Inc., New York.
Barrett J.T (1998). Microbiology and Immunology Concepts. Philadelphia, PA: Lippincott-Raven Publishers. USA.
Black, J.G. (2008). Microbiology: Principles and Explorations (7th ed.). Hoboken, NJ: J. Wiley & Sons.
Brian W.J Mahy and Mark H.C van Regenmortel (2010). Desk Encyclopedia of Human and Medical Virology. Elsevier Academic Press, San Diego, USA.
Brooks G.F., Butel J.S and Morse S.A (2004). Medical Microbiology, 23rd edition. McGraw Hill Publishers. USA.
Cann A.J (2011). Principles of Molecular Virology. Fifth edition. Academic Press, San Diego, United States.
Carter J and Saunders V (2013). Virology: Principles and Applications. Second edition. Wiley-Blackwell, New Jersey, United States.
Champoux J.J, Neidhardt F.C, Drew W.L and Plorde J.J (2004). Sherris Medical Microbiology: An Introduction to Infectious Diseases. 4th edition. McGraw Hill Companies Inc, USA.
Dimmock N (2015). Introduction to Modern Virology. Seventh edition. Wiley-Blackwell, New Jersey, United States.
Dimmock N.J, Easton A.J and Leppard K.N (2001). Introduction to modern virology. 5th edition. Blackwell Science publishers. Oxford, UK.
Discover more from #1 Microbiology Resource Hub
Subscribe to get the latest posts to your email.