Viable cell count: Viable cell count gives an estimate of the total number of living cells present in a given volume of a sample. Viable cell count can be determined by automated machines and with the use of counting chambers such as the haemocytometer (Figure 1) in the microbiology laboratory.
Haemocytometers are routinely used in the laboratory to determine viable cell count from samples and suspension of microbial cells in the laboratory.
Colony counts which involve the enumeration of the number of colonies on particular solid culture media can also be used to determine the viable counts of microbial cells. Pour plate methods and spread plate techniques are some colony counting techniques employed in the enumeration of microbial viable cell count.
Total cell count: Total cell count is an estimate of the total number of both living and dead cells in a given volume of a sample. It can be determined by direct counting methods using microscopy and other electronic instruments such as the coulter counter and turbidometry. Total cell counting techniques can be used to count bacteria, fungi (e.g. yeasts) and bacterial spores excluding viruses and viroids. Viruses are mainly counted using the electron microscope.
Optical density (OD): Optical density is a measure of the decrease in transmitted light when light is passed through a microbial suspension. It is usually expressed mathematically as OD=log10 (Io/I). “Io” is the intensity of the incident light while “I” is the intensity of the transmitted light. The technique of determining the OD value of a given sample or microbial suspension in the microbiology laboratory is generally known as turbidometry or turbidimetry.
Turbidometry is the technique used to estimate the actual concentration of cells in a given suspension by passing a beam of light through the suspension (suspended in a cuvette). The intensity of the transmitted light passing through the suspended fluid is compared to a standard control in order to determine the OD value of the cells in the liquid.
Optical density is the measure of the amount of light absorbed by a bacterial suspension when evaluated in a spectophotometric cuvette or blank. The OD value of a given suspension or sample is evaluated using specialized type of instruments such as the spectrophotometer and turbidimeters which gives reading as absorbance. Absorbance reading is synonymous to optical density (OD) value.
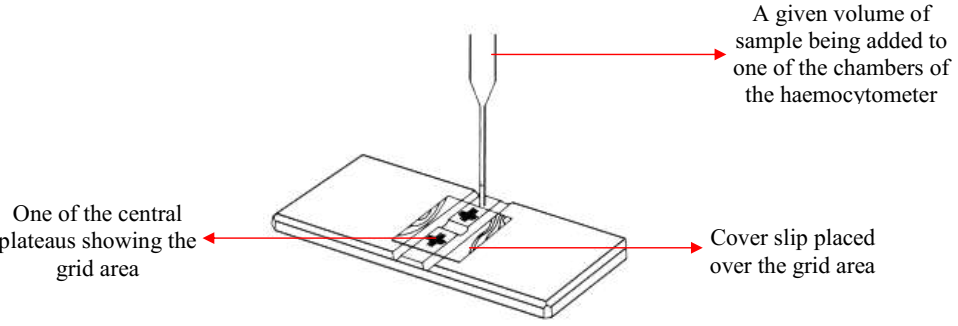
The haemocytometer is a rectangular glass block that consists of two central plateaus in which the samples is inoculated or added. The central plateaus are unique because they each consist of a square area that is divided into 400 small squares. Each of the small squares is 0.0025 mm2. A given volume of the sample or microbial suspension is added to the counting chamber (already covered with a cover slip) using the Pasteur’s pipette. The sample is drawn into the counting chamber by capillary action unlike in other cases where a drop of the suspension is added to a glass slide before covering it with cover slip (e.g. in microscopy).
The haemocytometer is allowed for about 30 minutes before viewing under the microscope in order to allow the cells to settle into the central plateaus properly. The count of cells per unit volume of the sample can be calculated by multiplying the number of cells counted after examining the 400 small square areas with the diameter of each of the small square area (i.e. 0.0025 mm2). If the suspension or sample to be counted with the haemocytometer was diluted before counting under the microscope, the count obtained must be counted with the dilution factor in order to get the final count of the microbial cells in the sample.
Table 1. Techniques of enumerating bacterial cell numbers and cell mass
| Cell numbers | Cell mass |
| Direct microscopic counts using the Microscope & improved Neubauer counting chamber device. Both dead cells & viable cells are counted. | Measurement of chemical parameters of microbial cells (e.g. nucleic acids & protein molecules). |
| Plate counts. It involves the plating out or spreading of serially diluted bacterial culture on media plate. Viable cells are mainly counted here. This method gives the colony forming unit (CFU) value of the counted bacteria. | Measurement of physical parameters of microbial cells (e.g. dry weight of bacteria). |
| Electronic counting machines. This method is used to count eukaryotes & other large organisms including yeasts, protozoa & algae. Electronic counters count both dead cells & living cells. | Measurement of chemical activity of microbial cells (e.g. CO2 & O2 production or utilization). |
| Membrane filters technique. This method involve the direct count of microbial cells growing on specialized filters known as membrane filters – with pores small enough to hold bacteria colonies in a solid culture media. | Measurement of bacterial turbidity. Microbial growth can be evaluated by assessing their growth in a broth medium. This technique allows microbiologists to determine microbial mass by measuring the intensity of light directed or absorbed by a turbid growth of bacterial cells in a broth culture medium. A spectrophotometer is used to measure this growth; and growth readings are taken in terms of absorbance which is extrapolated and taken as the actual cell mass of the organism. Spectrophotometer specifically measures the optical density or turbidity of the growing bacterial cells in a broth culture medium. Increase in the turbidity of the broth culture indicates increase of the microbial cell mass in the growth medium, and this is proportional to the number of microorganism present in the growth vessel (both viable and dead cells). |
Many techniques exist for the measurement of microbial growth in terms of assessing microbial cell numbers and microbial cell mass. These methods as stipulated in Table 1 above helps the microbiologists to elucidate the doubling (generation) time of a microbial cell. As explained in Table 1, these techniques also allow the microbiologist to assess the actual amount or number of both viable and dead cells present in the growth medium. The type of measurement technique to be employed is usually dependent on the type of analysis the microbiologist seeks to undertake.
References
Brooks G.F., Butel J.S and Morse S.A (2004). Medical Microbiology, 23rd edition. McGraw Hill Publishers. USA. Pp. 248-260.
Madigan M.T., Martinko J.M., Dunlap P.V and Clark D.P (2009). Brock Biology of microorganisms. 12th edition. Pearson Benjamin Cummings Publishers. USA. Pp.795-796.
Prescott L.M., Harley J.P and Klein D.A (2005). Microbiology. 6th ed. McGraw Hill Publishers, USA. Pp. 296-299.
Ryan K, Ray C.G, Ahmed N, Drew W.L and Plorde J (2010). Sherris Medical Microbiology. Fifth edition. McGraw-Hill Publishers, USA.
Singleton P and Sainsbury D (1995). Dictionary of microbiology and molecular biology, 3rd ed. New York: John Wiley and Sons.
Talaro, Kathleen P (2005). Foundations in Microbiology. 5th edition. McGraw-Hill Companies Inc., New York, USA.
Discover more from #1 Microbiology Resource Hub
Subscribe to get the latest posts to your email.