The LAL test is applied in the testing of pharmaceuticals, medical devices, water, food, and air because of its high sensitivity and specificity for endotoxins of Gram negative bacteria. LAL test is used for the quality control of pharmaceutical/medical parenteral preparations. The observation that the haemolymph (blood)of the Horseshoe crab can form clot in the presence of bacterial endotoxins gave rise to the Limulus amoebocyte lysate (LAL) test.
The amoebocytes (a part of the haemolymph) from Limulus polyphemus (Horseshoe crab) coagulate when in contact with the Lipid A portion of Gram negative bacteria endotoxins due to an enzymatic reaction. The amoebocytes are the main components of the haemolymph of the Horseshow crab; and it is what is responsible for the coagulation of the haemolymph in Limulus polyphemus crabs.
The amoebocytes contain pro-coagulant enzymes that trigger a chain of reactions; and the final product of these chain reactions is a gel comprised of coagulated proteins. The enzymatic response is produced when the amoebocytes enter into contact with the endotoxins. This mechanism is often compared to the trypsin that also triggers a chain of reactions to finally form the thrombin (the agent responsible for the coagulation of blood in humans). The Limulus polyphemus crab is one of the animals that have survived on land since prehistoric time with origins that date back more than 200 million years; and this resistant animal experiences coagulation in its haemolymph due to the presence of bacterial endotoxins.
The discovery of the mechanism behind this reaction led to the development of the LAL test – which is widely used today to test for the presence of bacterial endotoxins in a product or sample. The LAL test is specific and sensitive for the endotoxins from Gram negative bacteria. Despite their sensitivity and specificity for endotoxins of Gram negative bacteria, the LAL test is limited – because it cannot detect exotoxins of Gram positive bacteria and cellular components of viruses and fungi.
Thus, the LAL test could give a false positive test and false negative test since it is an in vitro test that detects endotoxins from Gram negative bacteria. In some quarters, the LAL test is usually combined with the in vivo (rabbit) test to detect microbial endotoxins in products. However, the rabbit test is gradually being refined and replaced with in vitro testing such as the LAL test and other in vitro tests that does not include the use of animals for research.
The rabbit test used to be a gold standard for the detection of bacterial endotoxins in parenteral products but the irregular and unpredictable body temperature of rabbits and other laboratory animals involved in this in vivo test has seen it being replaced by more specific and sensitive in vitro testing techniques like the LAL test. The rabbit test usually involves a measurement of the body temperature of the rabbit after the inoculation (injection) of not more than 10 ml/kg body weight of the test sample to be tested into the rabbit.
The body weight and temperature conditions of the rabbit are usually taken and recorded prior to and after injection of the test substance. A rise in the body temperature of the test rabbit after certain measured time intervals is indicative of a positive test result; and this implies that the test sample contained pyrogens – which caused the rise in the body temperature of the animal. The handling of the animal during the experiment and the possible hyperthermia (high body temperature) that could result with the rabbit test gives a false positive result.
The age, gender and housing conditions of the rabbit could also affect the outcome of the rabbit test result. An alternative to the rabbit test (in vivo test for bacterial endotoxins) is the LAL in vitro testing technique. The LAL test is only valid for detecting endotoxins of Gram negative bacteria and not any other type of pyrogens. Bacterial endotoxins (lipopolysaccharides) that can be detected using the LAL test are the pyrogens that pose the greatest safety risk for patients by causing fever, shock and death. Several techniques exist for carrying out the LAL test.
These methods include the LAL gel clot method, the turbidity assay method and the kinetic chromogenic LAL test. The use of human whole blood and ELISA technique are also used for detecting the presence of pyrogens in a given product. The kinetic chromogenic LAL test is most sensitive of all the LAL tests. It is performed with the help of incubation photometer and dedicated software. Kinetic Chromogenic LAL test is less affected by inhibitory products; and it is very well suitable for testing vaccines, antibiotics, other biological products.
The LAL test is an alternative method to the rabbit (in vivo) pyrogen test focused on detection of pyrogenic substances in sterile parenteral drugs. The principle behind the LAL test is based on the observation that when an endotoxin contacts clot proteins from circulating amoebocytes of Horseshoe crab (Limulus polyphemus), a clot forms. It is an in vitro test performed to check for the presence endotoxin in sample; and commercial LAL quantification test kits are available in the market for detecting the presence of bacterial endotoxins in pharmaceutical or medical products (Figure 1).
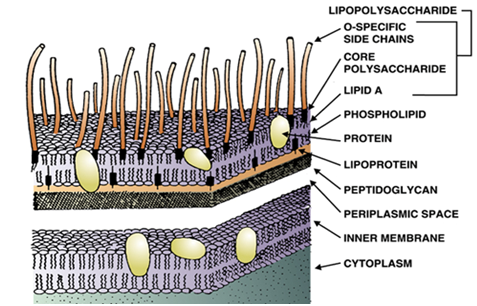
The LAL test measures the coagulation of the amoebocytes of the Horseshoe crab, initiated by the cell wall components (LPS) of Gram-negative bacteria with a molecular weight of > 8000 daltons. Smaller LPS component of bacterial endotoxins including those of Gram positive bacteria cannot be detected by the LAL test. The LAL test is critical in ensuring the quality control of parenteral drugs and other medical and pharmaceutical products because pyrogens pose a life-threatening risk of hypotensive shock to patients administered with contaminated drugs.
Biomedical companies bleed Horseshoe crabs (Figure 2) in a laboratory environment to obtain the Limulus amoebocyte lysate (LAL). The Limulus polyphemus belongs to a group of Horseshoe Crabs, which live in the Atlantic coast in the northern part of the American continent, including the Gulf of Mexico. The horseshoe crab is a marine organism that has inevitable importance in medicine and pharmaceutical companies.

The Horseshoe crabs are washed to remove sand and other marine debris from their exoskeletons; and those horseshoe crabs without visible injuries are placed on a bleeding rack and bled by puncturing the heart with a large gauge needle (Figure 3). On average, 30 % of the crab’s blood is removed before the wound clots naturally. The blood is placed in a centrifuge to separate the amoebocytes from the blue haemolymph that comprises the supernatant. Distilled water is then added to the separated amoebocytes; and the added water will eventually cause the cells to burst, or lyse. Clotting proteins inside the cells are released and separated from the rest of the solution; and the collected proteins are further processed into the powdered LAL product used for carrying out the LAL test. The Horseshoe Crabs are generally returned to the water within 72 hours of bleeding.

The amoebocytes are the main components of the blue haemolymph of the Horseshow crab; and it is what is responsible for the coagulation of the haemolymph in Limulus polyphemus crabs. The blue haemolymph (arrow) is the blood of the Horseshoe crab; and it is extracted from the animal and used for the commercial production of LAL test kits for the detection of pyrogens in contaminated pharmaceutical or medical samples. LAL is used in medical and pharmaceutical and/or laboratory detection kits to test products (especially those meant for parenteral administration) for the presence of Gram negative bacteria endotoxins and bacterial substances that can cause fevers in humans.
References
Arora D.R (2004). Quality assurance in microbiology. Indian J Med Microbiol, 22:81-86.
Ashutosh Kar (2008). Pharmaceutical Microbiology, 1st edition. New Age International Publishers: New Delhi, India.
Axelsen P.H (2002). Essentials of antimicrobial pharmacology. Humana Press, Totowa, New
Block S.S (2001). Disinfection, sterilization and preservation. 5th edition. Lippincott Williams & Wilkins, Philadelphia and London.
Denyer S.P., Hodges N.A and Gorman S.P (2004). Pharmaceutical Microbiology. 7th ed. Blackwell Publishing Company, USA.
Ejikeugwu Chika, Iroha Ifeanyichukwu, Adikwu Michael and Esimone Charles (2013). Susceptibility and Detection of Extended Spectrum β-Lactamase Enzymes from Otitis Media Pathogens. American Journal of Infectious Diseases. 9(1):24-29.
Ejikeugwu Chika, Iroha Ifeanyichukwu, Adikwu Michael and Esimone Charles (2013). Susceptibility and Detection of Extended Spectrum β-Lactamase Enzymes from Otitis Media Pathogens. American Journal of Infectious Diseases. 9(1):24-29.
Nally J.D (Ed.) (2007). Good manufacturing practices for pharmaceuticals. Sixth edition. Informa Healthcare USA, Inc, New York.
Discover more from Microbiology Class
Subscribe to get the latest posts sent to your email.