A pyrogen is simply defined as a fever-causing (inducing) agent that includes toxins of microorganisms. The phrase “pyrogens” is derived from the Greek word “Pyros” to mean “Fire”. Pyrogens are the lipopolysaccharide (LPS) component or endotoxins of bacteria especially Gram negative organisms. They also include the cell wall components of both Gram negative and Gram positive bacteria capable of inducing fever in human or animal hosts. Endotoxins are part of the outer membrane of the cell wall of Gram-negative bacteria; and they are invariably associated with Gram-negative bacteria whether the organisms are pathogenic or not. LPS also known as bacterial endotoxin and found in the cell membrane of Gram negative bacteria is the best studied pyrogen of bacteria.
LPS are the main components of the cell wall or cell membrane of Gram negative bacteria; and they are generally pyrogenic in nature. They are very heat-stable in nature and therefore are not easily destroyed under normal sterilization conditions. Bacterial endotoxins or LPS are ubiquitous in nature and can be found in the air, water, in the laboratory and even at work environments. Human activities and/or occupation that leads to the production and release of infectious particles containing microbes such as dust can make pyrogens to become airborne – through which possible human contamination or infection can occur.
Good manufacturing practice (GMP) requirements for the production of sterile products requires that sterile pharmaceutical or medical products be free from particulate matter, microbiological contamination and pyrogens in particular. Pyrogens could be parts of microbial cells including parts of bacteria, fungi and viruses; and these parts of microbial cells are of immense medical importance because of the untoward reactions such as fever and shock that they can cause in the human body.
The pyrogenic principles of bacteria especially Gram negative bacteria are usually attributable to some heat-stable substances secreted by these organisms; and which if found in parenteral drugs could induce fever (a rise in the body temperature of the host taking the medication). This phenomenon necessitates the need to continuously test and detect the presence of pyrogens in intravenous medications and other parenterals so that the batch of the products containing fever-inducing agents could be stopped from reaching the general public. Fever as we know it is one of the major symptoms or clinical signs of an infectious disease including those caused by viruses, fungi, protozoa and bacteria. The notable pyrogenic substances are usually the endotoxins of Gram negative bacteria especially the LPS component of Gram negative bacteria cell wall.
Other pyrogenic substances include exotoxins from Gram positive bacteria such as Streptococcus species and enterotoxins from Staphylococcus aureus.The lipoteichoic acid (LTA) components of Gram positive bacteria are also pyrogenic in nature (i.e. they are fever-inducing agents). There are basically two types of pyrogens viz: exogenous pyrogens and endogenous pyrogens. Exogenous pyrogens are endotoxins or LPS of Gram negative bacteria that induce fever in animal or human host when administered intravenously.
Endogenous pyrogens are neither endotoxins nor LPS, but they are fever-inducing agents that arise in the body of a host when exogenous pyrogens come in contact with certain host cell molecules such as monocytes or macrophages. Endogenous pyrogens are pyrogens generated by the host body; and they have potent inflammatory and pyrogenic effects in the body.
Exogenous pyrogens originate outside the body and induce temperature elevations when injected into humans and animals. Examples of exogenous pyrogens include LPS, microbes, microbial components of Gram negative bacteria, fungi, viruses, Gram positive bacteria, and non-microbial components such as drugs and steroids. Endogenous pyrogens are the primary mediators of fever in humans and animals; and they are homogenous substances produced internally in the body of a human or animal host. They are usually produced in the host in response to external stimuli (exogenous pyrogens).
Typical examples of endogenous pyrogens include interleukins, tumor necrosis factor (TNF) and platelet activating factor. Cytokines and prostaglandins are typical examples of endogenous pyrogens generated by the host body. Both endogenous and exogenous pyrogens cause a rise in the body temperature of the host. However, exogenous pyrogens are more clinically/medically significant than the endogenous pyrogens since the former (i.e. the exogenous pyrogens) enter the body of the host from the outside (especially through the administration of contaminated parenteral drugs).
Pyrogens particularly LPS of Gram negative bacteria provoke an immune response in their host by producing endogenous pyrogens including prostaglandins and inflammatory cytokines such as tumor necrosis factor (TNF) and interleukins. These chemical messengers when released in the body of a human host can transmit signals to the hypothalamus of the brain, to elevate the body temperature; and this rise in body temperature (i.e. fever) could result in septic shock and possibly death if not managed effectively.
Cytokines are proteinous substances released by the cells of the lymphatic (lymph) system, and they are directly involved in controlling our body’s response to inflammation. It is however noteworthy that cytokines are usually the first messenger molecules to be released from the macrophages when our body is exposed to pyrogenic substances such as LPS. Thus, cytokines can be used as markers of infection in a human or animal host. A rise in the body’s temperature (as mediated by the activities of the cytokines) is one of the mechanisms used by cytokines to defend the body against microbial infection. And this type of defense is innate immunity and usually not specific in its action.
The production of cytokines by the macrophages are usually proportionate to the amount of bacterial endotoxins that invaded the host; and thus the more inflammation-inducing substances (pyrogens) there are in the host’s body, the more cytokines are produced. Hence, the reaction can be used to quantify the inflammation-inducing potential of a given sample suspected of containing pyrogens.
Bacterial endotoxins and/or LPS of Gram negative bacteria stimulates the host macrophages (which are professional antigen presenting cells) to release inflammatory cytokines as aforementioned; and the excessive inflammation caused in the host as a result of the release of these chemical messengers could cause multiple organ failures and death. Thus pyrogenicity is an important aspect of medical and pharmaceutical sector – owing to the significance of pyrogens in causing infection or fever in human hosts. Pyrogenicity is simply defined as the ability of a pyrogen to cause infection or disease. The endotoxins of Gram negative bacteria consist of three different morphological regions (Figure 1).
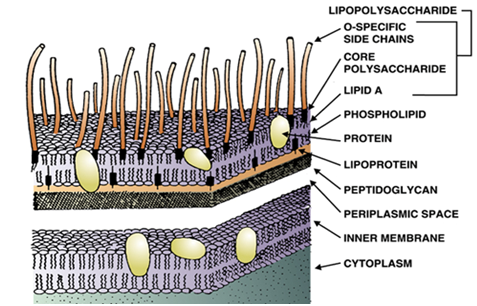
These regions include the Lipid A (a lipid moiety) region, the polysaccharide core and the O-(specific) antigenic side chains. The Lipid A region is linked to the polysaccharide core which in turn is linked to the O-(specific) antigenic side chains. Lipid A is the sole portion of the Gram negative bacterial cell wall that is responsible for the antigenic activity of LPS in the body of a human or animal host. The functions of bacterial endotoxins in a human host include the induction of fever, production of cytokines and prostaglandins, induction of hypotensive shock and possibly death. To this day, parenteral drugs including vaccines, drugs (antibiotics) and even some food products are regularly tested for the presence ofexogenous pyrogens – so that they can be confirmed as safe for human consumption.
Parenteral drugs and other medical/pharmaceutical products meant for systemic administration must be free from every form of microbial contamination and toxic/chemical substances capable of inducing fever (aside other medical conditions associated with pyrogens) in a human or animal host. And this is why the preparation and/or production processes for the production of parenteral products meant for medical/pharmaceutical usage is often carried out in sterile and/or aseptic conditions – so that the contamination of these products will be limited as much as possible. Thus, all the processes involved in the production of medical/pharmaceutical products intended for parenteral usage must be designed and handled in such a way that they eliminate the contamination of the production processes and/or equipments and instruments by potential and harmful microorganisms. It is critical to measure and detect the presence of pyrogens from parenteral drugs including water for injections prior to their usage in order to prevent adverse effects associated with pyrogens.
The presence of pyrogens in a product such as a parenteral drug can lead to fever, shock, hypotension (low blood pressure), organ failure and possibly death. Parenteral drugs including vaccines meant for systemic administration must be of a pyrogen-free quality before it can be certified safe for human consumption.
Pyrogen test is defined as a test that detects the presence of bacterial endotoxins (lipopolysaccharides) in a given product or sample including food, air, parenteral drugs and other pharmaceutical or medical products and devices. Such tests form part of the aspects of the quality control of these products; and it is critical to conduct pyrogen test on them prior to their release into the market for human or animal consumption.
Pyrogen testing can be carried out in two different ways which include in vitro pyrogen testing and in vivo pyrogen testing. While the in vitro pyrogen testing is carried out outside a living system (i.e. in a test plate or card) using antigenic substances, the in vivo pyrogen testing is normally carried out in a living system such as in a laboratory mouse or rabbit. The Limulus Amoebocyte Lysate (LAL) test is a typical example of an in vitro pyrogen testing that is widely used for detecting the presence of pyrogens in parenteral drugs.
References
Arora D.R (2004). Quality assurance in microbiology. Indian J Med Microbiol, 22:81-86.
Ashutosh Kar (2008). Pharmaceutical Microbiology, 1st edition. New Age International Publishers: New Delhi, India.
Axelsen P.H (2002). Essentials of antimicrobial pharmacology. Humana Press, Totowa, New
Block S.S (2001). Disinfection, sterilization and preservation. 5th edition. Lippincott Williams & Wilkins, Philadelphia and London.
Denyer S.P., Hodges N.A and Gorman S.P (2004). Pharmaceutical Microbiology. 7th ed. Blackwell Publishing Company, USA.
Nally J.D (Ed.) (2007). Good manufacturing practices for pharmaceuticals. Sixth edition. Informa Healthcare USA, Inc, New York.
Discover more from #1 Microbiology Resource Hub
Subscribe to get the latest posts to your email.


