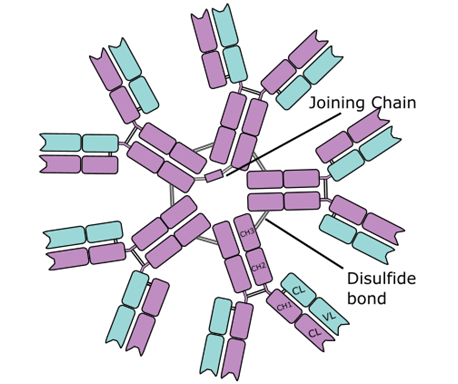
Immunoglobulin M (IgM) is an antibody that mainly exist as a pentamer i.e. it consists of five different monomeric units joined together by disulphide bonds and a joining (J) chain (Figure 1). The function of the J chain in the IgM structure is to help in the polymerization of the individual monomeric units that makeup the pentameric IgM antibody; and it does this via a sulphydryl residue close to the carboxyl terminal of the Fc region of the monomeric units.
It is the largest antibody out of the 5 isotypes of immunoglobulins; and IgM is often known as a macroglobulin because of its relatively higher molecular weight or size which is about 900,000 Daltons (Da). Because of its large size, IgM does not cross the placenta and it does not leave the blood stream where it gives the host protection against blood-borne infections (caused by both bacteria and viruses). Though the large size of IgM confines the antibody to the bloodstream where it provides protective functions against pathogenic microorganisms, IgM has a short lifespan and it is the first antibody to disappear from the blood serum.
Immunoglobulin M is the main antibody produced in primary immune response to an invading pathogen or antigen. IgM is also the first antibody to be produced in neonates or infants; and immunoglobulin M (a pentameric antibody) is the most efficient complement fixing antibody. This is because it has ten (10) antigen-binding sites unlike other isotypes of immunoglobulins; and IgM is more efficient in binding antigens with many antigenic determinant sites or epitopes (e.g. red blood cells and viral particles).
Thus IgM is a better complement fixation antibody, and it is very efficient in agglutination and in the cytolysis of microbial cells (e.g. bacteria). The complement system usually requires two adjacent Fc regions for complement activation; and this is provided by the pentameric structure of IgM; and this feature makes IgM a better complement fixation antibody than the other immunoglobulins. Increase in the production of IgM usually signifies an in utero infection in expectant mothers because it is the first immunoglobulin to be produced in neonates. IgM is usually present on the surfaces of all uncommitted or naïve B cells and it is the first immunoglobulin to be synthesized during B cell maturation in the bone marrow.

Immunoglobulin M is the first antibody to be produced during infection (i.e. in primary immune response to an antigen). The J chain helps IgM to bind to receptors on secretory mucosal cells; and this promote the entry of IgM into external body secretions (e.g. tears, saliva and breast milk). Each of the monomeric unit that make up the pentameric IgM structure are arranged with their Fc regions in the center of the pentamer while the 10 antigen-binding sites are arranged at the outside or periphery of the structure. IgA is another immunoglobulin apart from IgM that has a joining (J) chain.
REFERENCES
Abbas A.K, Lichtman A.H and Pillai S (2010). Cellular and Molecular Immunology. Sixth edition. Saunders Elsevier Inc, USA.
Actor J (2014). Introductory Immunology. First edition. Academic Press, USA.
Alberts B, Bray D, Johnson A, Lewis J, Raff M, Roberts K and Walter P (1998). Essential Cell Biology: An Introduction to the Molecular Biology of the Cell. Third edition. Garland Publishing Inc., New York.
Bach F and Sachs D (1987). Transplantation immunology. N. Engl. J. Med. 317(8):402-409.
Barrett J.T (1998). Microbiology and Immunology Concepts. Philadelphia, PA: Lippincott-Raven Publishers. USA.
Jaypal V (2007). Fundamentals of Medical Immunology. First edition. Jaypee Brothers Medical Publishers (P) Ltd, New Delhi, India.
John T.J and Samuel R (2000). Herd Immunity and Herd Effect: New Insights and Definitions. European Journal of Epidemiology, 16:601-606.
Levinson W (2010). Review of Medical Microbiology and Immunology. Twelfth edition. The McGraw-Hill Companies, USA.
Roitt I, Brostoff J and Male D (2001). Immunology. Sixth edition. Harcourt Publishers Limited, Spain.
Zon LI (1995). Developmental biology of hematopoiesis. Blood, 86(8): 2876–91.
Discover more from #1 Microbiology Resource Hub
Subscribe to get the latest posts to your email.