Cells of mammalian or prokaryotic or eukaryotic cells can be counted in the lab from samples using different kinds of counting chmabers. The neubauer counting chamber is one major type of counting chamber generally used for counting cells, manually in the microbiology laboratory. Improved neubauer counting chamber is a manual chamber or haemocytometer that allows cells to be counted in the laboratory. The counting of cells in a haemocytometer is an old technique of microbial cell enumeration in the laboratory; however, this old technique is still a valuable method to quantify the number of cells per mL in a suspension or sample.
AIM: To determine whether the cells found in a cerebrospinal fluid (CSF) specimen are pus cells or lymphocytes. And to count the white blood cells (WBCs) present in the CSF.
MATERIAL/APPARATUS: CSF specimen, microscope, cover slip, improved neubauer counting chamber, Pasteur pipette or bulb pipette, Turk’s solution, test tube test tube rack.
METHOD/PROCEDURE FOR CELL COUNT OF CSF SAMPLE
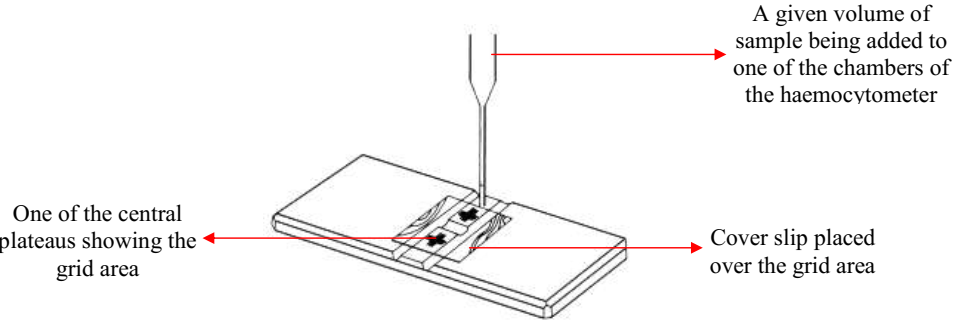
- Assemble the improved neubauer counting chamber (Figure 1), making sure that both the chamber and cover slip are completely clean.
- Place a clean cover slip on the top part of the neubauer counting chamber.
- Mix the CSF specimen. This is done by rotating the string used in obtaining it.
- Carefully fill the improved neubauer counting chamber with the CSF specimen, making sure not to allow the fluid to flow into the channels on each side of the chamber. This is done by allowing a drop of the CSF sample to touch the area where the cover slip makes contact with the neubauer counting chamber (Figure 2).
- Wait for about 2 minutes for the cells to settle.
- Focus the cells on the chamber under the microscope using the ×10 and ×40 objective lens. Make sure that the condenser iris of the microscope is sufficiently closed in order to give a good contrast.
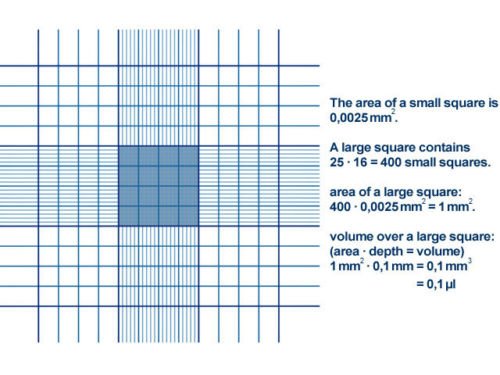
- Count the cells in any of the four (4) large squares or gridlines as indicated in the counting chamber (Figure 3).

NOTE: Use Turk’s solution to dilute bloody CSF specimen before filling the improved neubauer chamber. They are 2 ways to doing this dilution depending on the amount or concentration of the blood in the CSF.
- One in five dilutions (1:5): This dilution is made if the CSF specimen contains much blood. It is done by placing 4 drops of Turk’s solution plus 1 drop of the CSF specimen in a clean test tube.
- One in two dilutions (1:2): This dilution is made if the CSF specimen does not contain much blood. It is done by placing 1 drop of Turk’s solution plus 1 drop of the CSF specimen in a clean test tube.
NOTE: The essence of diluting a bloody CSF specimen with Turk’s solution before performing any analysis on the sample is to “lyse/breakdown the red blood cells (RBCs) in the CSF specimen”. The RBCs makes it unsuitable or difficult to count the white blood cells (WBCs) present in the bloody CSF specimen.
REPORTING OF THE RESULT: Report the appearance of the CSF specimen based on whether it is cloudy, clear, purulent or bloody as aforementioned. Count the white blood cells in any of the large squares of the improved neubauer chamber (Figure 3), and report the number of cells by multiplying the number of WBCs counted with the number of dilution made. This gives you an estimate of the number of white blood cells present in the sample.
An example on how to calculate the amount of WBCs is as follows:
- For 1 in 2 CSF dilutions: If for example you counted 200 WBCs, it will be 200 × 2 = 400. Report the cell count as: 400 × 106 cell/mm3
- For 1 in 4 CSF dilutions: If for example you counted 80 WBCs, it will be 80 × 4 = 320.
Report the cell count as: 320 × 106 cell/mm3/
References
Basic laboratory procedures in clinical bacteriology. World Health Organization (WHO), 1991. Available from WHO publications, 1211 Geneva, 27-Switzerland.
Beers M.H., Porter R.S., Jones T.V., Kaplan J.L and Berkwits M (2006). The Merck Manual of Diagnosis and Therapy. Eighteenth edition. Merck & Co., Inc, USA.
Biosafety in Microbiological and Biomedical Laboratories. 5th edition. U.S Department of Health and Human Services. Public Health Service. Center for Disease Control and Prevention. National Institute of Health. HHS Publication No. (CDC) 21-1112.2009.
Cheesbrough M (2010). District Laboratory Practice in Tropical Countries. Part I. 2nd edition. Cambridge University Press, UK.
Cheesbrough M (2010). District Laboratory Practice in Tropical Countries. Part 2. 2nd edition. Cambridge University Press, UK.
Collins C.H, Lyne P.M, Grange J.M and Falkinham J.O (2004). Collins and Lyne’s Microbiological Methods. Eight edition. Arnold publishers, New York, USA.
Disinfection and Sterilization. (1993). Laboratory Biosafety Manual (2nd ed., pp. 60-70). Geneva: WHO.
Garcia L.S (2010). Clinical Microbiology Procedures Handbook. Third edition. American Society of Microbiology Press, USA.
Garcia L.S (2014). Clinical Laboratory Management. First edition. American Society of Microbiology Press, USA.
Fleming, D. O., Richardson, J. H., Tulis, J. I. and Vesley, D. (eds) (1995). Laboratory Safety: Principles and practice. Washington DC: ASM press.
Dubey, R. C. and Maheshwari, D. K. (2004). Practical Microbiology. S.Chand and Company LTD, New Delhi, India. Gillespie S.H and Bamford K.B (2012). Medical Microbiology and Infection at a glance. 4th edition. Wiley-Blackwell Publishers, UK.
Discover more from #1 Microbiology Resource Hub
Subscribe to get the latest posts to your email.


