Cloning is a molecular biology technique which is used to make millions of copies of a particular gene of interest or a piece of DNA molecule. It generally involves taking a piece of DNA molecule (containing specific gene sequence of interest) from a donor organism where it naturally exists and putting same into a cloning host cell (e.g. Escherichia coli) through the help of a vehicle such as a vector which carries the gene of interest into the recipient host. At the end of a gene cloning process, many identical copies of the cloned DNA or gene known as clones will be generated for further molecular studies; and the cloned gene of interest also expresses or produces the proteins which it actually encodes within its host cell. A clone is a population of identical cells or DNA molecules.
Vectors in gene cloning technique are pieces of circular DNA molecule that are competent enough to transport DNA between hosts and are also capable of independent replication within the cells of their host organisms. A vector is a vehicle for foreign or passenger DNA molecules; and most vectors used for gene cloning techniques are either plasmids or phages. Cloning vectors therefore are DNA molecules that are used to transport cloned sequences of a gene or DNA between biological hosts and the test tubes.
Typical examples of cloning vectors used for gene cloning techniques and other recombinant DNA technological applications include plasmids, yeast artificial chromosomes (YAC), reporter gene vectors, bacteriophage lambda or phage, cosmid vectors and shuttle vectors amongst others. However, the type of cloning vector used for the gene cloning technique is usually dependent on the expertise of the researcher and the type of experimentation to be undertaken because each of these vectors varies in their ability to transport the gene of interest from one host or test tube to another.
Plasmids are mostly used in gene cloning techniques because they have small sizes and are easy to manipulate. Their circular structure makes them to be more stable than other vectors, and plasmids have biomarkers such as antibiotic resistance markers which aid their easy detection and recovery from a cloning process. The millions of copies of the cloned DNA or gene generated after a given gene cloning technique have a wide variety of medical and biomedical as well as industrial applications.
For example, copies of the cloned DNA can be used for sequencing analysis, molecular probes and for paternity tests. Cloned DNA or gene can also be used to generate transgenic animals and crops with immense resistance to pests; and cloned gene can also be used to study the gene expression and function of a given protein molecule in a living system. Several biopharmaceutical products (e.g. insulin) and proteins that are of medical significance have also been generated in large amounts through gene cloning techniques; and the significance of this important technique of molecular biology is enormous.
The term cloning encompasses gene cloning and molecular cloning. Gene cloning can be achieved in a variety of ways but gene cloning by PCR (in vitro technique) and cell-based gene cloning techniques (in vivo method) are usually the main methods involved in performing any cloning experiment in the molecular biology laboratory. While the cell-based gene cloning technique is mainly performed in a living system which may include a bacterial cell, yeast cell, mammalian cell or arthropods; the PCR gene cloning technique is carried out in a piece of test tube using the thermocycler. Cloning allows a copy of any specific part of a DNA or RNA sequence to be selected among many other copies and be reproduced in an unlimited amount in a host cell.
PROPERTIES OF PLASMIDS USED FOR GENE CLONING TECHNIQUES
- Plasmids contain selectable marker such as antibiotic resistance gene which aid in their easy detection and recovery after the gene cloning experiment.
- Plasmids used for gene cloning have relatively small sizes (e.g. 10 Kb), and thus they can be easily purified and manipulated. However, they cannot be used for the cloning of large sized DNA molecules (especially eukaryotic nucleic acid) whose sizes are above 10 Kb (e.g. 100 Kb). Such large DNA molecules are cloned with other vectors such as the YAC vector.
- Their circular shape or structure makes them more stable than other vectors used for gene cloning manipulations.
- Plasmids can produce several copies of the DNA insert unlike other vectors which are limited in their generated clones.
- They are capable of autonomous replication in vivo.
STEPS INVOLVED IN GENE CLONING
Gene cloning (both cell-based and in vitro technique) involves some series of procedures and these shall be highlighted in this unit.
- Isolation of plasmid DNA and chromosomal DNA molecule: Plasmid DNA isolation as well as the isolation of chromosomal DNA molecule is usually the first step in any gene cloning experiment. Several techniques are available for the isolation of plasmid DNA and chromosomal DNA from microbial cells. To isolate plasmid DNA molecule from a microbial cell, the bacteria containing the plasmid is first grown overnight in nutrient agar plates and incubated at the correct temperature and growth conditions. The growth medium is incorporated with the antibiotic that corresponds to the antibiotic resistance marker on the plasmid DNA to be isolated; and this allows only the bacteria that contain the plasmid to grow. Several methods exist for the isolation and purification of plasmid DNA molecule.
In the simplest method, the bacterial cells are lysed in test tube by incorporating lysozymes, RNase enzyme and a chelating agent (e.g. EDTA) to suspensions of the organism. The RNase enzyme degrades RNA molecules while the EDTA chelates divalent ions such as Mg++. Lysozyme is a cell wall degrading enzyme, and it attacks the peptidoglycan layer of the bacterial cell wall. The mixture is centrifuged in order to pellet large molecules. The plasmid DNA molecule is usually contained in the supernatant which also contain other soluble cell components.
The supernatant in this case is known as the cell lysate. The lysate is treated with NaOH to denature all DNA molecules; and a column resin technique which normally binds DNA molecules is used to purify the plasmid DNA molecule. In the column resin method, small amounts of the resin are mixed with the supernatant containing the plasmid DNA molecule. Plasmid DNA binds to the resin. Plasmid-bound resin is collected in a small column; and the plasmid DNA is eluted from the resin while the remaining cell components are washed away.
Several rapid techniques are available for the isolation of plasmid DNA molecule from cells. The isolation of chromosomal DNA molecule from cells is similar to the procedure used for the isolation of plasmid DNA molecule. However, chromosomal DNA molecule unlike the plasmid DNA is precipitated; and the cell lysate is extracted with phenol. Chromosomal DNA is much fragile than plasmid DNA and thus, the former cannot be purified using columns as is the case for plasmids. Centrifugation technique is use obtain the precipitated chromosomal DNA molecule.
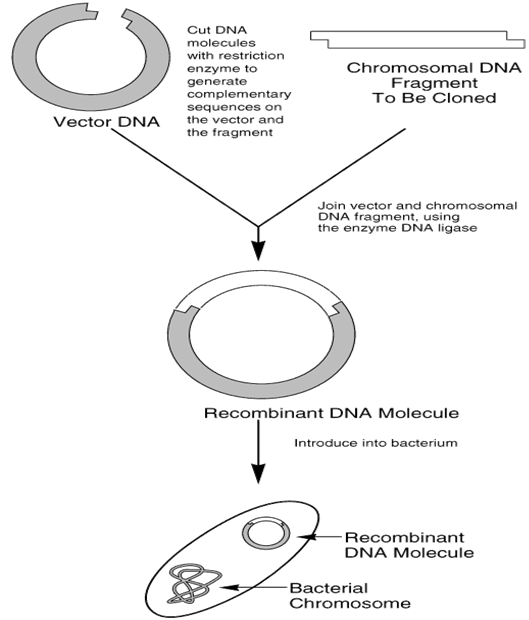
- Cutting of DNA or gene of interest and vector: The gene of interest or DNA is cut or nicked with specific restriction enzyme or endonuclease in vitro. The same restriction enzyme used in cutting the DNA of interest is also used to cut the plasmid vector that will carry the gene of interest or DNA into the host cell. The restriction enzyme specifically cut the plasmid vector at one of its restriction sites in order to allow for the insertion of the foreign or exogenous DNA. The reason for using the same restriction enzyme to cut both the DNA and plasmid vector is to ensure specificity of nicking so that the foreign DNA can be properly inserted. Complementary sequences on the DNA fragment and the plasmid vector will be generated after cutting (Figure 1).
- Ligation: After nicking the DNA molecule and plasmid vector respectively, both are mixed in a test tube where DNA ligase enzyme is added for ligation. Ligation is the molecular biology technique of joining two DNA molecules together with the help of DNA ligase enzyme. After ligation, a recombinant DNA molecule carrying the gene of interest to be cloned will be formed (Figure 1). The restriction enzyme used for cutting should be inactivated before the addition of DNA ligase enzyme for ligation activity in order to prevent the endonuclease from cutting the DNA molecule every time it is joined by the DNA ligase enzyme.
- Isolation and selection: Plasmids used for gene cloning techniques normally contain selectable biomarkers such as antibiotic resistance genes. And by isolating and plating them onto agar plate containing the antibiotic to which the organism is resistant to, only successfully transformed bacterial cells which have taken up the plasmid will be able to grow. The bacterial cells are resistant to the antibiotic in the agar medium because they harbour plasmid that expresses a particular antibiotic resistance gene.
- Expression: The inserted and cloned gene of interest is transcribed and translated into proteins. Thus the gene of interest is therefore expressed within the host cell with the gene product being a specific protein molecule. After cloning a gene, it is important to have the cloned gene express the product it encodes. The cloned gene can also be sequenced to know the exact sequence of bases in the DNA molecule.
Gene cloning in general terms involves the reproduction or making of several copies of the gene of an individual organism through molecular biology techniques (e.g. PCR). The gene of interest is cut with a specific restriction enzyme (endonuclease) and it is inserted into a vector (cut with the same endonuclease) which acts as a vehicle and transports the gene of interest into the host cell (e.g. a bacterial cell). But before then, the cut gene of interest or DNA is joined with the vector (e.g. plasmid) using DNA ligase; and this forms a recombinant DNA (rDNA) molecule.
The rDNA molecule is inserted into the host cell where it is further replicated as the chromosomal DNA of the host organism is being replicated. Specific protein molecule which the gene of interest encodes will be produced and expressed in the host cell. Note: The gene cloning process can be visualized by the gel electrophoresis technique; and this enables the researcher to monitor the reactions going on in the test tube where most of the reaction is taking place.
References
Alberts B, Bray D, Lewis J, Raff M, Roberts K and Watson J.D (2002). The molecular Biology of the Cell. Fourth edition. New York, Garland, USA.
Chen I and Dubnau D (2004). DNA uptake during bacterial transformation. Nat. Rev. Microbiol. 2 (3): 241–249.
Cooper G.M and Hausman R.E (2004). The cell: A Molecular Approach. Third edition. ASM Press.
Dale J (2003). Molecular genetics of bacteria. Jeremy W. Dale and Simon Park (4th eds.). John Wiley & Sons Ltd, West Sussex, UK. Pp. 312-313.
Das H.K (2010). Textbook of Biotechnology. Fourth edition. Wiley edition. Wiley India Pvt, Ltd, New Delhi, India.
Lewis R (2004). Human Genetics: Concepts and Applications. Sixth edition. McGraw Hill Publishers, USA.
Lodish H, Berk A, Matsudaira P, Kaiser C.A, Kreiger M, Scott M.P, Zipursky S.L and Darnell J (2004). Molecular Cell Biology. Fifth edition. Scientific American Books, Freeman, New York, USA.
Madigan M.T., Martinko J.M., Dunlap P.V and Clark D.P (2009). Brock Biology of Microorganisms, 12th edition. Pearson Benjamin Cummings Inc, USA.
McPherson M and Moller S (2002). PCR: The Basics. 2nd edition. Taylor and Francis Group. New York, USA.
Sambrook, J., Russell, D.W. (2001). Molecular Cloning: a Laboratory Manual, 3rd edn. Cold Spring Harbor Laboratory Press, New York.
Synder L, Peters J.E, Henkin T.M and Champness W (2013). Molecular Genetics of Bacteria. Fourth edition. American Society of Microbiology Press, USA.
Tamarin Robert H (2002). Principles of Genetics. Seventh edition. Tata McGraw-Hill Publishing Co Ltd, Delhi.
Discover more from #1 Microbiology Resource Hub
Subscribe to get the latest posts to your email.