Extended spectrum beta-lactamases (ESBLs) are beta-lactamase enzymes that breakdown or hydrolyze broad-spectrum beta-lactam drugs especially the third-generation oxyimino-cephalosporins (e.g. ceftazidime, ceftriaxone and cefotaxime). There is no consensus for the actually definition of ESBLs; but the acronym “ESBL” was originally coined to reflect the expanded substrate spectrum of the earlier beta-lactamase enzymes (e.g. TEM- and SHV-enzymes). ESBLs are transmissible beta-lactamases that are plasmid-mediated, and can be inhibited by beta-lactamase inhibitors such as clavulanic acid, tazobactam or sulbactam. Typically, ESBLs arise by mutations that alter the amino acid configuration around the active site of the earlier beta-lactamases (i.e. TEM and SHV enzymes); and they can be transmitted to susceptible bacteria through genetic elements such as transposons and plasmids.
Several ESBL types exist and they include the TEM-1 and SHV-5 ESBL types. TEM is the acronym for “Temoneira”, the name of the first patient from whom a beta-lactamase producing bacterium was originally isolated from in Europe; and SHV stands for sulphydryl group or variant and it denotes a variable response of an organism to sulphydryl inhibitors. Of particular importance is another group ofnon-mutant ESBLs(generally known as CTX-M-type ESBLs) that did not arose by mutation but by the acquisition of resistant plasmids (especially beta-lactam genes) from some obscure species such as Kluyvera species which is a non-pathogenic commensal organism.
CTX-M type ESBLs (e.g. CTX-M-15) unlike the mutant TEM- and SHV- type ESBLs (that arose by mutation in the amino-acid sequence of the earlier beta-lactamases) were so named because of their greater activity against the cephalosporin, cefotaxime. ESBLs carries tremendous clinical implications since most of the drugs (or antibiotics) used in therapy against bacterial infections especially those that are beta-lactam built are rendered inactive by them. The clinical implications of ESBL producing bacteria is huge since beta-lactam drugs (penicillins and cephalosporins inclusive) are the most widely used antibiotics in clinical medicine; and if ESBLs are resistant to all of them, it then means that there is a limitation of drugs available for the treatment of some bacterial-related infections.
Some of the risk factors for acquisition of ESBL-producing bacterial pathogens include: prior usage of antibiotics especially the cephalosporins, increased length of stay in the hospital, , increased severity of microbial infection, and prolonged use of invasive medical devices such as catheters and feeding tubes. Though the first reported case of ESBL infection was in Europe (particularly in Germany) in 1983, ESBL-producing bacteria now occur worldwide and they have since spread from one region to another and even in the community. ESBLs are found amongst strains of Enterobacteriaceae especially Escherichia coli, Klebsiella pneumoniae, and Klebsiella oxytoca. But they have also been reported in some non-enteric organisms such as Acinetobacter baumannii and Pseudomonas aeruginosa.
The accurate detection of ESBL producing bacteria from clinically important specimens and especially from patients whose antimicrobial therapy is not effective is imperative in order to avoid clinical failure due to inappropriate antimicrobial therapy. Such measures will also help in forestalling the emergence and spread of the pathogen in both the hospital environment and in the community. Pathogenic bacteria especially E. coli and Klebsiella species that produce ESBL are multidrug resistant organisms that extend their resistance to some non-beta-lactam drugs including fluoroquinolones and aminoglycosides but the best choice of treatment for an ESBL is usually the carbapenems (e.g. imipenem and meropenem).
The Clinical Laboratory Standard Institute, CLSI (formerly National Committee for Clinical Laboratory Standards, NCCLS) have come up with a number of guidelines for the accurate detection and reporting of organisms producing ESBLs; and these measures include a series of phenotypic and genotypic tests which determine ESBL phenotypes and beta-lactamase resistant genes in pathogenic bacteria suspected to produce these broad-spectrum antibiotic degrading enzymes. Because the routine antimicrobial susceptibility testing (AST) methods carried out in most hospital laboratories are not capable of detecting ESBL resistance without some modifications, the CLSI recommends the use of any of the 3rd-generation oxyimino-cephalosporins (e.g. ceftriaxone, cefotaxime and ceftazidime) to screen for the presence of an ESBL (Table 1); and organisms found to show reduced susceptibility according to the CLSI breakpoints should be recommended for a phenotypic confirmatory test such as the double disk synergy test (DDST) method (Table 2).
Table 1. ESBL Screening Breakpoints according to the CLSI criteria
| Disk | Content (µg) | Resistance (BP, mm) | Susceptible (BP, mm) | ESBL Screening BP, (mm) |
| Cefpodoxime | 10 | 17 | 27 | 17 |
| Ceftazidime | 30 | 14 | 18 | 22 |
| Cefotaxime | 30 | 14 | 23 | 27 |
| Ceftriaxone | 30 | 13 | 21 | 25 |
| Aztreonam | 30 | 15 | 22 | 27 |
Table 2. ESBL phenotypic confirmatory test
| DISCS | INTERPRETATION |
| Ceftazidime 30 mg Ceftazidime – clavulanic acid 30/10 mg AND Cefotaxime 30 mg Cefotaxime – clavulanic acid 30/10 mg | A ≥ 5 mm increase in zone diameter for EITHER antibiotic (i.e. ceftazidime & cefotaxime) tested in combination with clavulanic acid versus its zone when tested alone confirms ESBL production. |
Polymerase chain reaction (PCR) can also be employed to amplify specific ESBL genes in putative ESBL-producing bacteria. The template for the phenotypic detection of ESBL production in clinical isolates in the microbiology laboratory is shown in Figure 1.

KEY: CTX = Cefotaxime (30 µg): AMC = Amoxycillin-clavulanic acid (20/10 µg): CAZ = Ceftazidime (30 µg)
ESBL positive bacteria produce a characteristic shape known as the keyhole effect in vitro on culture media plate; and this feature which is caused by the activity of a beta-lactamase inhibitor (e.g. clavulanic acid) is usually used to infer the presence of an ESBL phenotypically (Figure 2).
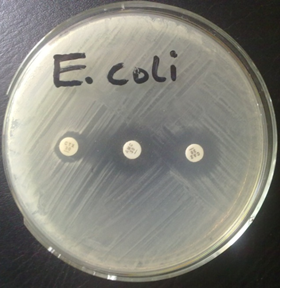
The DDST technique is performed by inoculating a Mueller-Hinton (MH) agar plate with the test bacteria (adjusted to 0.5 McFarland turbidity standards) as per the CLSI criteria. The MH plate is allowed to dry for about 5 minutes and a central disk (amoxicillin-clavulanic acid) is placed on the plate. Third generation cephalosporins (e.g. ceftazidime and cefotaxime) are placed 15 mm away from the central disk, and the plates incubated at 37oC for 24 hrs. DDST is inferred to be positive if there is an enlargement of the zones of inhibition (> 5 mm) of any of the cephalosporins. The zone of inhibition of cephalosporins is normally enhanced in the presence of a beta-lactamase inhibitor (in this case, amoxicillin-clavulanic acid).
Antibiotic disks of amoxycillin-clavulanic acid (20/10 µg) is placed at the center of a Mueller-Hinton (MH) agar plate, and antibiotic disks containing cefotaxime (30 µg) and ceftazidime (30 µg) is each placed at a distance of 15 mm (center to enter) from the central disc, amoxycillin-clavulanic acid 20/10 µg (as shown in Figure 1). Incubate the plates in the incubator at 37oC for 18-24 hrs. ESBL production is inferred phenotypically when the zones of inhibition of the cephalosporins (cefotaxime 30 µg and ceftazidime 30 µg) were expanded by the amoxycillin-clavulanic acid disk (20/10 µg), thus showing a key hole effect. A ≥ 5 mm increase in the inhibition zone diameter for either of the cephalosporins (ceftazidime and cefotaxime) tested in combination with amoxycillin-clavulanic acid versus its zone when tested alone confirms ESBL production phenotypically.
References
Arora D.R (2004). Quality assurance in microbiology. Indian J Med Microbiol, 22:81-86.
Ashutosh Kar (2008). Pharmaceutical Microbiology, 1st edition. New Age International Publishers: New Delhi, India.
Axelsen P.H (2002). Essentials of antimicrobial pharmacology. Humana Press, Totowa, New Jersey, USA. Al-Jasser A.M (2006). Extended – Spectrum Beta – Lactamases (ESBLs): A Global Problem. Kuwait Medical Journal, 38(3):171-185.
Bisht R., Katiyar A., Singh R and Mittal P (2009). Antibiotic Resistance – A Global Issue of Concern. Asian Journal of Pharmaceutical and Clinical Research, 2 (2):34-39.
Block S.S (2001). Disinfection, sterilization and preservation. 5th edition. Lippincott Williams & Wilkins, Philadelphia and London.
Cars O and Nordberg P (2005). Antibiotic resistance: The faceless threat. International Journal of Risk & Safety in Medicine, 17 (3/4): 103-110.
Carson C.F., Hammer K.A and Riley T.V (2006). Malaleuca alternifolia (Tea Tree) oil: A Review of Antimicrobial and other Medicinal Properties. Clinical Microbiology Review, 19(1):50-62.
Cowan M.M (1999). Plant products as antimicrobial agents. Clinical Microbiology Reviews., 564-582.
Denyer S.P., Hodges N.A and Gorman S.P (2004). Pharmaceutical Microbiology. 7th ed. Blackwell Publishing Company, USA.
Ejikeugwu Chika, Ikegbunam Moses, Ugwu Chigozie, Eze Peter, Iroha Ifeanyichukwu, and Esimone Charles (2013). Phenotypic Detection of Klebsiella pneumoniae Strains – Producing Extended Spectrum β-Lactamase (ESBL) Enzymes. Scholars Academic Journal of Biosciences. 1(1):20-23.
Ejikeugwu Chika, Iroha Ifeanyichukwu, Adikwu Michael and Esimone Charles (2013). Susceptibility and Detection of Extended Spectrum β-Lactamase Enzymes from Otitis Media Pathogens. American Journal of Infectious Diseases. 9(1):24-29.
Ejikeugwu Chika, Iroha Ifeanyichukwu, Adikwu Michael and Esimone Charles (2013). Susceptibility and Detection of Extended Spectrum β-Lactamase Enzymes from Otitis Media Pathogens. American Journal of Infectious Diseases. 9(1):24-29.
Finch R.G, Greenwood D, Norrby R and Whitley R (2002). Antibiotic and chemotherapy, 8th edition. Churchill Livingstone, London and Edinburg.
Joslyn, L. J. (2000). Sterilization by Heat. In S. S. Block (Ed.), Disinfection, Sterilization, and Preservation (5th ed., pp. 695-728). Philadelphia, USA: Lippincott Williams and Wilkins.
Lai P.K and Roy J (2004). Antimicrobial and chemopreventive properties of herbs and spices. Curr. Med. Chem, 11 (11): 1451–1460.
Livermore D.M (2004). The need for new antibiotics. Clinical Microbiology & Infection, 4(10): 1-9.
Mascaretti O.A (2003). Bacteria versus antibacterial agents: An integrated approach. Washington: ASM Press.
Nally J.D (Ed.) (2007). Good manufacturing practices for pharmaceuticals. Sixth edition. Informa Healthcare USA, Inc, New York.
Discover more from #1 Microbiology Resource Hub
Subscribe to get the latest posts to your email.


