AIM: To isolate organism from cerebrospinal fluid (CSF) specimen as an aid in the diagnosis of cerebrospinal meningitis and other microbial infection of the CSF.
MATERIAL/APPARATUS: Cerebrospinal fluid (CSF) specimen, blood agar (BA), chocolate agar (CA), Bunsen burner, inoculating loop, incubator, anaerobic jar, MacConkey agar (MCA).
NOTE: The CSF specimen should be collected only by an experienced medical officer, physician or health worker. A lumber puncture is required to obtain the CSF specimen and should be done aseptically to avoid contamination. This is very important so as to avoid introducing any contaminant into the CSF – which is a sterile fluid of the human body.
CSF is free from normal microbial flora and thus its collection and handling in the laboratory should be done aseptically. The examination/culture of CSF specimen should not be delayed like other specimens, and the results of tests should be immediately reported to the physician as soon as they become available. If the CSF specimen is from a newborn patient, MacConkey agar (MAC) should be used in addition with the blood agar (BA) and chocolate agar (CA).
MAC and BA plates should be incubated aerobically while the CA plate should be incubated anaerobically. The CSF specimen usually comes to the microbiology laboratory in the syringe used for its collection, thus primary sample inoculants required for the inoculation of the culture media should be obtained by releasing a drop on each of the culture media plates used for the process.
METHOD/PROCEDURE FOR CSF CULTURE
- Label the CA and BA plates with the patient’s name and laboratory number.
- Inoculate the CSF specimen on each of the CA and BA plates.
- Incubate the BA plate in the incubator at 37oC overnight.
- Incubate the CA plate in the anaerobic jar at 37oC overnight.
- After incubation, examine both plates for significant bacteria growth.
- Subculture the isolated organisms onto freshly prepared culture media plates (e.g. CA and BA) for the isolation of pure cultures.
- Perform biochemical testing on significant bacterial isolates.
- Perform antimicrobial susceptibility testing only when a significant bacterium is isolated.
REPORTING OF THE RESULT: Look especially for colonies that could be Escherichia coli or other coliform in the MAC plate and report. Look particularly for Neisseria meningitides, Streptococcus pneumonia, and Haemophilus influenzae on both the CA and BA plates and report appropriately (Figure 1).
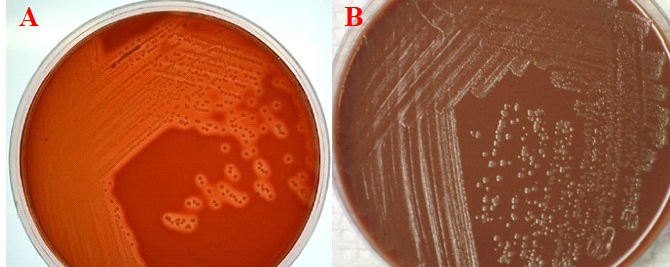
References
Basic laboratory procedures in clinical bacteriology. World Health Organization (WHO), 1991. Available from WHO publications, 1211 Geneva, 27-Switzerland.
Beers M.H., Porter R.S., Jones T.V., Kaplan J.L and Berkwits M (2006). The Merck Manual of Diagnosis and Therapy. Eighteenth edition. Merck & Co., Inc, USA.
Biosafety in Microbiological and Biomedical Laboratories. 5th edition. U.S Department of Health and Human Services. Public Health Service. Center for Disease Control and Prevention. National Institute of Health. HHS Publication No. (CDC) 21-1112.2009.
Cheesbrough M (2010). District Laboratory Practice in Tropical Countries. Part I. 2nd edition. Cambridge University Press, UK.
Cheesbrough M (2010). District Laboratory Practice in Tropical Countries. Part 2. 2nd edition. Cambridge University Press, UK.
Collins C.H, Lyne P.M, Grange J.M and Falkinham J.O (2004). Collins and Lyne’s Microbiological Methods. Eight edition. Arnold publishers, New York, USA.
Disinfection and Sterilization. (1993). Laboratory Biosafety Manual (2nd ed., pp. 60-70). Geneva: WHO.
Garcia L.S (2010). Clinical Microbiology Procedures Handbook. Third edition. American Society of Microbiology Press, USA.
Garcia L.S (2014). Clinical Laboratory Management. First edition. American Society of Microbiology Press, USA.
Fleming, D. O., Richardson, J. H., Tulis, J. I. and Vesley, D. (eds) (1995). Laboratory Safety: Principles and practice. Washington DC: ASM press.
Dubey, R. C. and Maheshwari, D. K. (2004). Practical Microbiology. S.Chand and Company LTD, New Delhi, India.
Gillespie S.H and Bamford K.B (2012). Medical Microbiology and Infection at a glance. 4th edition. Wiley-Blackwell Publishers, UK.
Discover more from #1 Microbiology Resource Hub
Subscribe to get the latest posts to your email.


