The CAMP test (named for the original authors: Christie, Atkins, and Munch-Petersen) was first used in the identification of group B streptococci (GBS). Group B streptococci secrete a protein called CAMP factor or “protein B” that interacts with the beta-hemolysin produced and secreted by Staphylococcus aureus, this results in enhanced or synergistic hemolysis.
The CAMP test is a test to identify group B β-hemolytic streptococci (Streptococcus agalactiae) based on their formation of a substance (CAMP factor) that enlarges the area of hemolysis formed by the β-hemolysin elaborated from Staphylococcus aureus.
CAMP test is one of the most affordable, easy-to-perform methods that may be used in clinical laboratories to identify Listeria monocytogenes (Lm) and Streptococcus agalactiae (group B streptococcus, GBS) from clinical samples.
The term “CAMP” comes from the initials of authors (Christie, Atkins, and Munch-Petersen) who first studied this assay as well as the particular phenomenon it is based on; we mean that test positivity is typically indicated by formation of an arrow-shaped hemolysis (‘arrowhead’) where GBS and Lm grow in a zone of Staphylococcus aureus (Sa) β-hemolysin activity perpendicularly to Sa and without touching.
CAMP test is a diagnostic tool that reliably and quickly provides presumptive identification of GBS and Lm. Arrowheads promptly develop when bacterial inocula are in an early stage of growth and the sheep blood plate is prewarmed to 37°C.
CAMP test detects the production of diffusible, extracellular protein known as CAMP factor. CAMP factor is produced by Group B Streptococcus (GBS) such as S. agalactiae. The CAMP factor acts synergistically with the beta lysin produced by Staphylococcus aureus to produce a zone of enhanced lysis of sheep or bovine red blood cells. CAMP test depend on the elaboration of two toxins during growth to form a typical arrowhead at the junction of the two organisms (i.e., the GBS and the S. aureus isolates) when they are placed perpendicular to each other.
Procedure
- Streak a beta-lysin–producing strain of S. aureus down the center of a sheep blood agar plate.
- The test organism streak should be 3 to 4 cm long.
- Streak test organisms across the plate perpendicular to the S. aureus streak within 2 mm. (Multiple organisms can be tested on a single plate).
- Incubate at 35°-37°C in ambient air for 18-24 hours.
- Wedge shaped pattern radiating from the test organism near the S. aureus indicates a positive test result (Figure 1).
Result interpretation
A positive result is indicated by the presence of a clear zone (arc or circle) of enhanced hemolysis. Enhanced hemolysis only where the diffused, slight hemolysis overlaps is considered a positive reaction.
A negative result will show no areas of enhanced hemolysis near the colony in the presence of the CAMP Spot Test Reagent.
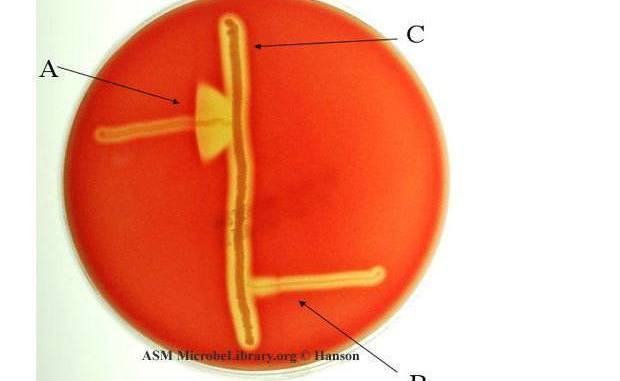
This is an example of a positive CAMP test indicated by the formation of an arrowhead where the Strep group B (Streptococcus agalactiae) meets the Staphylococcus aureus (light-yellow/golden middle streak).
Though CAMP test is used generally to detect Group B Streptococci (GBS) such as Streptococcus agalactiae; CAMP test can also be used for the detection of Listeria monocytogenes. Of the Listeria species, L. monocytogenes is the only one that has been clearly documented as a human pathogen and is one of the few species of Listeria that is CAMP factor positive.
LIMITATIONS
- The CAMP test should not be used alone, but rather, in combination with the appropriate colony morphology, gram reaction, and other biochemical tests on colonies from pure culture for complete identification.
- Extended incubation times or elevated incubation temperatures may give false-positive results.
- Interpretation of the CAMP spot test can be affected by excessive agar depth. Plate depth of approximately 1.5mm has been recommended.
- It is recommended that CAMP spot test be used in conjunction with the Centers for Disease Control (CDC) protocol for processing clinical specimens when testing for group B streptococci.
- Use sheep blood agar plates only. Human, horse, rabbit, or guinea pig blood plates will not give a proper reaction.
- L. ivanovii only shows a positive CAMP reaction when using an alternative CAMP test method, in which Rhodococcus equi replaces S. aureus.
- A small percentage of group A streptococci will have a positive CAMP reaction.The test should only be performed on colonies that have the morphology of group B streptococci (gray to translucent colonies which have a narrow zone of beta-hemolysis with a gram stain and catalase reaction indicative of streptococci). The PYR Test (Cat no. Z75) may be used to further differentiate group A streptococci from group B.
- Colonies of Listeria monocytogenes have a narrow zone of beta-hemolysis on sheep blood agar and may be confused with group B beta-hemolytic streptococci, if catalase and gram stain are not performed.
- The presence of beta-antitoxin in some batches of sheep blood will inhibit staphylococcal beta-toxin production; therefore QC of CAMP Spot Test Reagent is recommended each lot of sheep blood agar. The user should verify that the manufacturer has tested each lot of media for the CAMP reaction, or alternatively, perform quality control in-house for each lot of media.
- Only colonies that have been growing for at least 18 hours should be tested with the spot test. Colonies only 12 hours old can give false-negative results, presumably because the colony may not yet have produced adequate amounts of CAMP factor in the synergistic hemolysis. Also, if the test is performed on GBS colonies that are on sheep blood agar that have been incubated for more than 48 hours, the zone of synergistic hemolysis may be more difficult to interpret if darkening of the blood medium has occurred.
References
Jorgensen., et al. Manual of Clinical Microbiology, American Society for Microbiology, Washington, D.C.
Tille, P., et al. Bailey and Scott’s Diagnostic Microbiology, C.V. Mosby Company, St. Louis, MO.
Isenberg, H.D. Clinical Microbiology Procedures Handbook, Vol. I, II & III. American Society for Microbiology, Washington, D.C.
Koneman, E.W., et al. Color Atlas and Textbook of Diagnostic Microbiology, J.B. Lippincott Company, Philadelphia, PA.
Anderson, N.L., et al. Cumitech 3B; Quality Systems in the Clinical Microbiology Laboratory, Coordinating ed., A.S. Weissfeld. American Society for Microbiology, Washington, D.C.
MacFaddin, J.F. Biochemical Tests for Identification of Medical Bacteria,, Lipincott Williams & Wilkins, Philadelphia, PA.
Abbreviated Identification of Bacteria and Yeast; Approved Guideline, M35-A. 2002. Clinical Laboratory Standards Institute (CLSI – formerly NCCLS), Villanova, PA.
Discover more from #1 Microbiology Resource Hub
Subscribe to get the latest posts to your email.


