Biochemical tests are series of experiments that can be used to differentiate bacteria based on their metabolic activity or ability to utilize a given substrate such as glucose. These tests are generally useful in characterizing bacteria in the Microbiology Laboratory, and they take advantage of the organism’s physiological, osmotic, and nutritional requirements. The identification of the invading bacterial pathogen in a particular clinical disease condition is critical to the survival of the affected patients. This is because the quick and accurate identification of the bacterial species implicated in the disease condition will automatically lead to a better understanding of the disease process and the subsequent direction of the effective therapy to eliminate it.
A general knowledge of the normal flora that naturally inhabits the human body is indispensable in the diagnosis of bacterial-related diseases, thus the reason to biochemically test suspected bacterial isolates from cultures and ensure that treatment is not directed at commensal organisms but only against pathogenic strains. Pathogenic microbes must be differentiated from commensals or normal microflora in clinical specimens after culture, and one way of doing this is to subject the isolated bacteria to a series of biochemical tests – which help laboratory scientist to different a pathogen from a non-pathogen. Bacteriological findings including biochemical tests must be able to properly distinguish invasive bacterial pathogens from indigenous microorganisms (i.e. normal floras).
Biochemical tests can also help to different even closely related bacterial species. For example, pathogenic S. aureuscan be differentiated from non-pathogenic S. aureusin the laboratory because the former (pathogenic S. aureus) produces coagulase enzymes that cause blood plasma to clot while the latter (non-pathogenic S. aureus) does not produce this enzyme, and thus cannot cause blood plasma to clot. Certain bacterial species have the innate ability to elaborate or express definite enzymes and substances which are used in their own characterization in the Microbiology Laboratory. Colonies of bacteria growing on an agar plate differ characteristically in their shapes and appearances. Some bacteria also produce specific odour which are also used in their characterization.
Bacterial species also differ in their staining properties when exposed to certain dyes, and thus Gram staining and other available staining techniques can as well be used to differentiate one bacterium from another. Bacterial species also differ in their nutritional requirement for growth, thus some selective/differential culture media (e.g. Mannitol salt agar, MacConkey agar) can presumptively help to identify bacteria and even distinguish amongst closely related species. By growing bacteria on various culture media plates, microbiologists determine the key growth requirements of the respective organism in addition to other criteria which are all dependent on the biochemical and metabolic activity of the bacteria.
Biochemical tests assist microbiologists to quickly and accurately identify bacteria from clinical or environmental samples. Many of these tests are commercially available as test kits, and they can help a microbiologist to run as many biochemical tests as possible on a given sample at a relatively short period unlike some conventional biochemical tests that require an overnight incubation before a result can be obtained. Though quick and accurate in identifying bacterial species from either clinical or environmental samples, the series of available commercial systems used for the rapid identification of bacteria are often expensive and difficult to obtain in some developing countries.
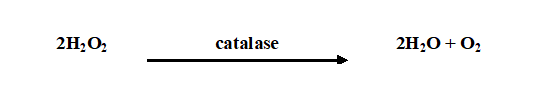
Generally, biochemical tests help microbiologists to simply put a name to a particular microorganism, and settle all uncertainty surrounding its identification especially if the isolated bacterium is from samples suspected to contain mixed bacterial cultures. Some commonly available conventional biochemical tests used for the identification of bacteria from either clinical or environmental samples include coagulase test, indole test, oxidase test, citrate test, catalase test, sugar utilization test, nitrate reduction test, VP test, urease test, triple sugar iron test, morphological test, starch hydrolysis test, acid production test, hydrogen sulphide test, blood agar haemolysis test and satellitism test. The biochemical tests used for the identification of Gram negative bacteria is usually different from those used for the identification of Gram positive bacteria (Table 1).
Table 1. Summary of some biochemical tests and culture media used for identifying bacteria
| Gram Positive Bacteria | Gram Negative Bacteria |
| Voges-Proskauer (VP) test | |
| Coagulase test | Urease test |
| Catalase test | Oxidase test |
| Mannitol Salt Agar (MSA) plates | MacConkey agar plates |
| Blood Agar (BA) plates | Simmon’s Citrate Agar (SCA) plates |
| Optochin sensitivity test | Triple Sugar Iron Agar (TSIA) |
| Bacitracin sensitivity test | Sugar utilization test |
| Starch hydrolysis test | Methyl red test |
| DNase test | Indole test |
| Bile solubility test | Nitrate utilization test (also for Gram positive bacteria) |
| CAMP test | Eosin Methylene Blue (EMB) Agar |
| Key: cAMP = Cyclic Adenosine Mono-Phosphate |
References
Basic laboratory procedures in clinical bacteriology. World Health Organization (WHO), 1991. Available from WHO publications, 1211 Geneva, 27-Switzerland.
Beers M.H., Porter R.S., Jones T.V., Kaplan J.L and Berkwits M (2006). The Merck Manual of Diagnosis and Therapy. Eighteenth edition. Merck & Co., Inc, USA.
Biosafety in Microbiological and Biomedical Laboratories. 5th edition. U.S Department of Health and Human Services. Public Health Service. Center for Disease Control and Prevention. National Institute of Health. HHS Publication No. (CDC) 21-1112.2009.
Cheesbrough M (2010). District Laboratory Practice in Tropical Countries. Part I. 2nd edition. Cambridge University Press, UK.
Cheesbrough M (2010). District Laboratory Practice in Tropical Countries. Part 2. 2nd edition. Cambridge University Press, UK.
Collins C.H, Lyne P.M, Grange J.M and Falkinham J.O (2004). Collins and Lyne’s Microbiological Methods. Eight edition. Arnold publishers, New York, USA.
Disinfection and Sterilization. (1993). Laboratory Biosafety Manual (2nd ed., pp. 60-70). Geneva: WHO.
Garcia L.S (2010). Clinical Microbiology Procedures Handbook. Third edition. American Society of Microbiology Press, USA.
Garcia L.S (2014). Clinical Laboratory Management. First edition. American Society of Microbiology Press, USA.
Fleming, D. O., Richardson, J. H., Tulis, J. I. and Vesley, D. (eds) (1995). Laboratory Safety: Principles and practice. Washington DC: ASM press.
Dubey, R. C. and Maheshwari, D. K. (2004). Practical Microbiology. S.Chand and Company LTD, New Delhi, India.
Gillespie S.H and Bamford K.B (2012). Medical Microbiology and Infection at a glance. 4th edition. Wiley-Blackwell Publishers, UK.
Discover more from Microbiology Class
Subscribe to get the latest posts sent to your email.