Gel electrophoresis is one type of electrophoresis technique, and its procedure is highlighted below. The following materials and steps and steps are employed in gel electrophoresis technique:
- Agarose gel is used for performing gel electrophoresis in the microbiology or molecular biology laboratory. It is noteworthy that the agar powder used for gel electrophoresis is different from the powdered agar used for the preparation of routine culture media plates for microbial cultivation. In gel electrophoresis, agarose gel powder is used to prepare the gel. The agarose gel is prepared by mixing a particular amount of agarose powder (e.g. 1.5 %) in a buffer solution or deionized water. Agarose gel could be made with varying concentrations of agarose ranging between 0.6 % – 3 %; and this usually depends on the size of the nucleic acid fragments the researcher wishes to resolve or separate.
- Larger fragments of nucleic acids are separated or resolved better in a gel with a lower percentage of agarose while smaller nucleic acid fragments are separated better in a gel with a higher percentage of agarose. To prepare 1.5 % agarose gel for example, measure out 1.5 g of agarose powder and dissolve same in 100 ml buffer or deionized water in a conical flask. Stir the mixture properly to break up all clumps; and heat the mixture by boiling at a particular temperature (e.g. 1 – 2 min) in a microwave oven until the solution becomes clear as water.
- A Bunsen burner flame could also be improvised for heating the agarose solution in places where microwave oven is unavailable. The agarose gel solution should be heated until a homogenous solution is formed. After heating, the homogenate gel should be allowed to cool to about 60oC before pouring gel onto the gel casting apparatus or slab. Agarose, a white powder and the buffer solution are the two basic components of an agarose gel; and both needed to be heated sufficiently to make the gel required to run the gel electrophoresis technique.
- A toothed comb (Figure 1) is used to form wells known as sample wells in the agarose gel. Thus, the toothed comb should be placed into the gel casting apparatus or tray prior to pouring of the gel so that the wells will be formed appropriately. The toothed comb is removed prior to the insertion of the DNA samples into the wells. The number of wells or holes formed is usually dependent on the number of samples or organisms to be analyzed; and thus the type of toothed comb used in agarose gel experimentation varies. Samples for gel electrophoresis analysis are individually inoculated or dispensed into each of the toothed wells using micropipette (Figure 2). Multiple pipette tips (Figure 3) also exist for multiple analyses during molecular biology experimentation.

- Dispense the cooled homogenous solution into the gel casting apparatus or tray (Figure 4). The pouring should be done slowly, and all air bubbles formed during the pouring should be removed using a disposable pipette. Air bubbles could generally affect the shape of the wells if allowed to settle around the comb.
- The poured gel is allowed in the gel casting apparatus for some minutes (e.g. 20 mins) so that it will set or gel to form agarose gel slab. The gel casting apparatus gives the poured gel its characteristic horizontal shape required for agarose gel electrophoresis technique. Once cooled and gelled, the gel is now ready for agarose gel electrophoresis experimentation. It is then inserted into the electrophoretic matrix or chamber in which a buffered solution is also added to. In practice, the agarose gel slab is submerged in the buffered solution in the electrophoretic tank.
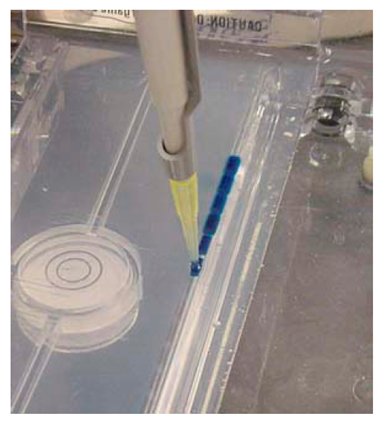
- Pipette the individual samples into the sample wells created in the agarose gel by the comb (Figure 5). Ensure that the pipette tip is changed for each sample to be pipetted. A DNA fragment or ladder (with known or standard size) is added in one of the wells (usually the first well); and the ladder is used to compare the separated DNA fragments (with unknown sizes).
In some agarose gel experimentation, ethidium bromide (EtBr) solution is added alongside the DNA solution to be analyzed. However, the EtBr is usually added to the prepared gel after cooling and before pouring onto the gel electrophoresis tank.
EtBr act as a chemical staining agent which helps to visualize the DNA bands or fragments after the electrophoresis experimentation. (EtBr is a dye that binds to DNA and clearly marks the position of the individual DNA fragments). In some agarose gel experimentation, the staining dye (in this case EtBr) is not added alongside the DNA solution to be electrophoresed.
But it is added prior to or after the electrophoresis analysis since its main function is to aid the visualization of the DNA fragments. Note: EtBr is mutagenic or carcinogenic in nature, and thus should be handled with care. SYBR Green, a nucleic acid gel stain is another staining agent that could be used in gel electrophoresis technique to visualize separated nucleic acid fragments.
However, EtBr solution is the most commonly used dye in gel electrophoresis experimentations; and it is critical that the researcher wears gloves when handling EtBr since the dye is a mutagen and could easily be absorbed by the skin to cause health problems in the individual.
- An electric current (e.g. 100 volts) is passed through the gel; and the process is allowed to run for the appropriate time limit. DNA, a negatively charged molecule moves from the negatively charged electrode (cathode) towards the anode (positive electrode). The DNA moves through the gel matrix, smaller molecules move faster than the larger molecules. The electric charge or current is switched off once the electrophoresis process is completed.
- Separated DNA fragments is visualized under UV light and photographed after soaking the gel slab in EtBr or any other staining dye.
References
Alberts B, Bray D, Lewis J, Raff M, Roberts K and Watson J.D (2002). The molecular Biology of the Cell. Fourth edition. New York, Garland, USA.
Chen I and Dubnau D (2004). DNA uptake during bacterial transformation. Nat. Rev. Microbiol. 2 (3): 241–249.
Cooper G.M and Hausman R.E (2004). The cell: A Molecular Approach. Third edition. ASM Press.
Dale J (2003). Molecular genetics of bacteria. Jeremy W. Dale and Simon Park (4th eds.). John Wiley & Sons Ltd, West Sussex, UK. Pp. 312-313.
Das H.K (2010). Textbook of Biotechnology. Fourth edition. Wiley edition. Wiley India Pvt, Ltd, New Delhi, India.
Lewis R (2004). Human Genetics: Concepts and Applications. Sixth edition. McGraw Hill Publishers, USA.
Lodish H, Berk A, Matsudaira P, Kaiser C.A, Kreiger M, Scott M.P, Zipursky S.L and Darnell J (2004). Molecular Cell Biology. Fifth edition. Scientific American Books, Freeman, New York, USA.
Madigan M.T., Martinko J.M., Dunlap P.V and Clark D.P (2009). Brock Biology of Microorganisms, 12th edition. Pearson Benjamin Cummings Inc, USA.
McPherson M and Moller S (2002). PCR: The Basics. 2nd edition. Taylor and Francis Group. New York, USA.
Sambrook, J., Russell, D.W. (2001). Molecular Cloning: a Laboratory Manual, 3rd edn. Cold Spring Harbor Laboratory Press, New York.
Synder L, Peters J.E, Henkin T.M and Champness W (2013). Molecular Genetics of Bacteria. Fourth edition. American Society of Microbiology Press, USA.
Tamarin Robert H (2002). Principles of Genetics. Seventh edition. Tata McGraw-Hill Publishing Co Ltd, Delhi.
Discover more from Microbiology Class
Subscribe to get the latest posts sent to your email.