Filtration is simply defined as the separation of particles from fluid or liquids by the use of a porous material that allows the passage of the fluid but stops or holdback unwanted particles from passing through it. Filtration can be used to separate microorganisms from a liquid or gas in which they are dispersed. It is a sterilization technique that can be used to sterilize heat-labile (sensitive) substances.
Heat-labile substances or liquids known to be free from viroids or viruses can be sterilized by passing them through membrane filters capable of holding back or stopping other microorganisms including bacteria and fungi. Filtration largely depends on a mechanical sieving action that suspends and holds larger particles and organisms that cannot pass through the pore size of the membrane filter.
Nevertheless, suspended particles and microorganisms may also be suspended or adsorbed to the surface of the filter as a result of some electrostatic forces; and this is because some membrane filters are highly adsorptive and thus can absorb particles suspended in a fluid via electrostatic action.
Membrane filtration technique is a technique that uses a physical barrier (usually a porous membrane or filter) to separate particles and microorganisms suspended in a fluid sample including food, air and water. The particles in the test samples are separated on the basis of their size and shape with the use of pressure and specially designed membrane filters with different pore sizes.
Membrane filters are microporous plastic films with specific pore size ratings. They are also known as screen, sieve or microporous filters; and membrane filters retain particles or microorganisms larger than their pore size primarily by surface capture.
Membrane filters are thin films made up of cellulose nitrate, cellulose acetate, nylon, polycarbonate or polyvinylidene materials. The main aim of the membrane filtration technique is to separate or concentrate substances in a fluid (liquid) sample. The membrane filtration technique is used to estimate or measure the amount of indicator organisms including feacal coliforms, total coliforms and Escherichia coli present in a sample.
It is an important technique generally applied in the examination of public health samples such as water and food. What makes the membrane filters a veritable tool in the microbiological analysis of environmental samples including food and water is the notable ability of the filter to permit the passage of large volumes of liquid or fluid while at the same time retaining particles of bacterial size on its surface.
The membrane filtration technique was introduced in the 1950s by Goetz and Tsuneishi – who published that cellulose nitrate and cellulose acetate membranes can be used as a means of capturing any bacterium present in a sample of water during filtration. Membrane filtration technique is an alternative to the most probable number (MPN) technique; and both methods are used for the estimation of bacteria and other microbes present in public health samples such as water, air and food.
The major advantage of the membrane filtration technique over the MPN technique is that the former isolates discrete colonies of bacteria whereas the latter only indicates the presence or absence of an approximate number of microorganisms present in a given sample (usually indicated by the formation of turbidity in the test tubes).
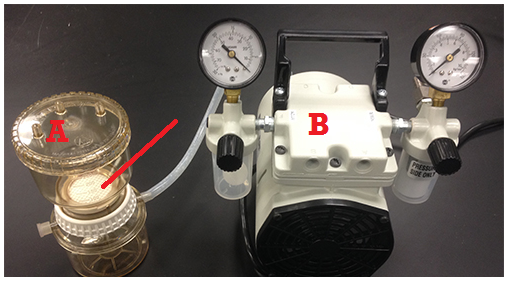
A 47 mm nitrocellulose membrane filters with a pore size of 0.45 mm are usually used for the membrane filtration technique (Figure 1). However, membrane filters with a variety of pore sizes are commercially available for various bacteriological and/or microbiological experiments. In the membrane filtration technique, a known volume of the test sample is filtered through a membrane filter and bacterial cells present in the sample are retained on the surface of the membrane filter (Figure 2).
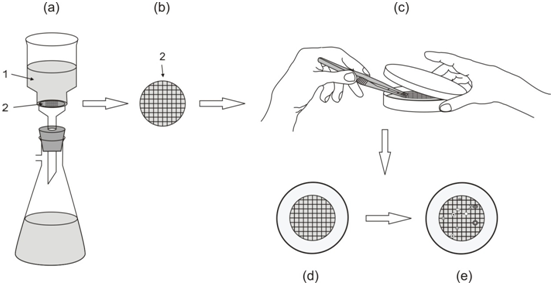
After filtration, the membrane filter is placed onto a Petri plate containing the suitable growth medium. Nutrient pad sets are commercially available for checking environmental samples for the presence of coliforms (Figure 3). These nutrient Pads are impregnated with dehydrated culture media in a Petri dish and membrane filters; and they are ready-to-use culture medium with specific pore size membrane filters.
During incubation, the nutrients diffuse through the filter from the growth medium and allow the development of bacterial colonies. Practically, the membrane filter is incubated on a suitable solid culture media plate; and incubated at the appropriate environmental conditions that favour bacterial growth.

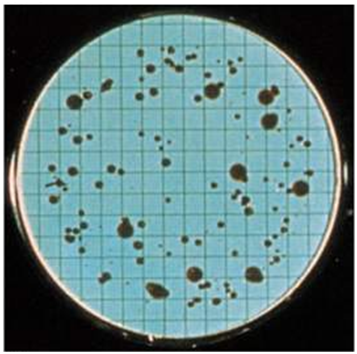
Bacterial cells trapped on the membrane filter will grow into visible colonies that can be counted, and a bacterial density can be calculated from these colonies (Figure 4). The number of bacterial colonies that grow on the membrane filter gives an indication of the number of bacteria present in the volume of sample filtered or passed through the membrane filter. The membrane filtration technique should be carried in an aseptic manner in order to prevent any microbial contamination of the process.
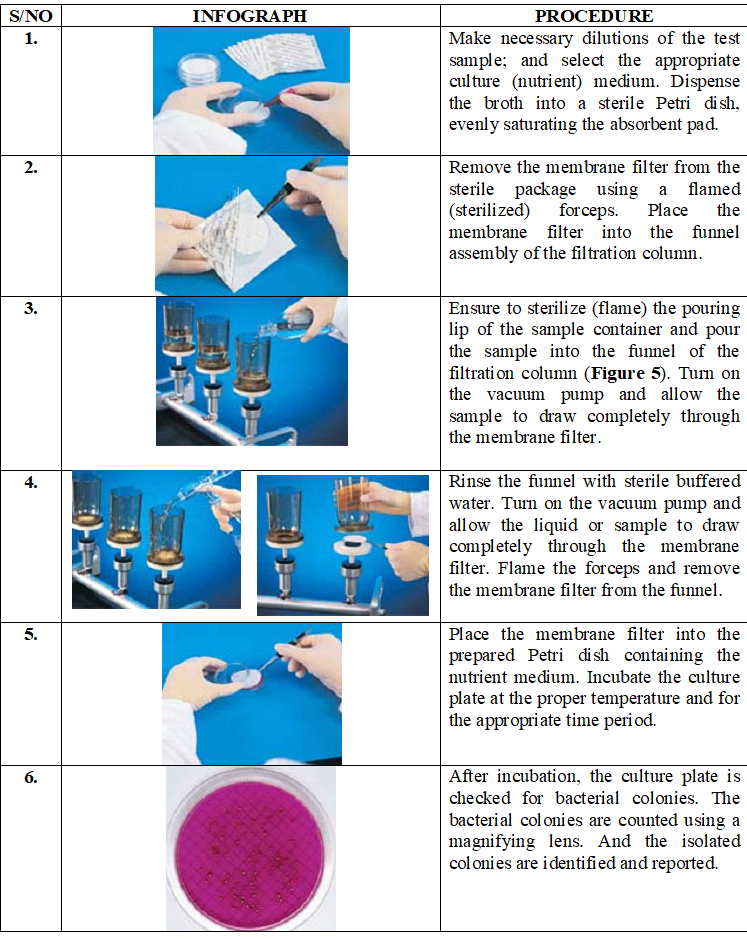
A step-by-step procedure of carrying out the membrane filtration technique in an aseptic manner to avoid contamination of the process is shown in Table 1. The bacterial count gives an estimation of the amount of the bacterial population present in the test samples; and the number of bacteria per unit volume is calculated from the number of colonies which develop on the membrane filter after incubation.
The Endo agar medium is usually used in the membrane filtration technique. Endo agar is both a selective and differential agar medium. Endo agar is a selective culture media because it contains sodium deoxycholate and sodium Lauryl sulphate – which inhibit the growth of Gram positive bacteria. Colonies of lactose fermenting bacteria such as E. coli produces pink to red colonies with a metallic sheen on the Endo agar; and this makes the medium a differential culture medium. Membrane filtration technique is applied in many areas including food and beverage industries and even in water treatment plants. It is also applied in determining or monitoring the quality of air, food and water. Membrane filtration technique is also applied in the pharmaceutical and cosmetic industries especially for the monitoring of water used for production for the presence of Pseudomonas aeruginosa.
Table 1. Step-by-step procedure of carrying out the membrane filtration technique
ADVANTAGES OF MEMBRANE FILTRATION TECHNIQUE
- Membrane filtration technique is a fast and simple way to estimate bacterial population in a sample.
- Large sample volumes can be tested using the membrane filtration technique.
- The preparation time in membrane filtration technique is reduced when compared to other traditional methods of estimating bacteria such as the MPN technique.
- Membrane filtration technique allows for the isolation and enumeration of discrete bacterial colonies.
- Membrane filtration techniques provide results on bacterial count within 24 hours unlike the MPN technique that takes several days to conclude.
References
Abrahams P.W (2006). Soil, geography and human disease: a critical review of the importance of medical cartography. Progress in Physical Geography, 30:490-512.
Ahring B.K, Angelidaki I and Johansen K (1992). Anaerobic treatment of manure together with industrial waste. Water Sci. Technol, 30, 241–249.
Andersson L and Rydberg L (1988). Trends in nutrient and oxygen conditions within the Kattegat: effects on local nutrient supply. Estuar. Coast. Shelf Sci, 26:559–579.
Ballantyne A.P, Alden C.B, Miller J.B, Tans P.P and White J.W.C (2012). Increase in observed net carbon dioxide uptake by land and oceans during the past 50 years. Nature, 488: 70-72.
Baumgardner D.J (2012). Soil-related bacterial and fungal infections. J Am Board Fam Med, 25:734-744.
Bennett E.M, Carpenter S.R and Caraco N.F (2001). Human impact on erodable phosphorus and eutrophication: a global perspective. BioScience, 51:227–234.
Bunting B.T. and Lundberg J (1995). The humus profile-concept, class and reality. Geoderma, 40:17–36.
Chang S.T (2006). The world mushroom industry: trends and technological development. International J. Medicinal Mushrooms, 8:297-314.
Jee C and Shagufta (2007). Environmental Biotechnology. APH Publishing Corporation, Darya Ganj, New Delhi, India.
Maier R.M, Pepper I.L. and Gerba C.P (2000). Environmental Microbiology. Academic Press, San Diego.
Miguel A, Manuel F, Francisco J.P and Antonio B (2006). Environmental biocatalysis: from remediation with enzymes to novel green processes. TRENDS in Biotechnology, 24(6):1-7.
Mishra B.B, Nanda D.R and Dave S.R (2009). Environmental Microbiology. First edition. APH Publishing Corporation, Ansari Road, Darya Ganj, New Delhi, India.
Paul E.A (2007). Soil Microbiology, ecology and biochemistry. 3rd edition. Oxford: Elsevier Publications, New York.
Pelczar M.J Jr, Chan E.C.S, Krieg N.R (1993). Microbiology: Concepts and Applications. McGraw-Hill, USA.
Pelczar M.J., Chan E.C.S. and Krieg N.R. (2003). Microbiology of Soil. Microbiology, 5th Edition. Tata McGraw-Hill Publishing Company Limited, New Delhi, India.
Pepper I.L and Gerba C.P (2005). Environmental Microbiology: A Laboratory Manual. Second Edition. Elsevier Academic Press, New York, USA.
Roberto P. Anitori (2012). Extremophiles: Microbiology and Biotechnology. First edition. Caister Academic Press, Norfolk, England.
Salyers A.A and Whitt D.D (2001). Microbiology: diversity, disease, and the environment. Fitzgerald Science Press Inc. Maryland, USA.
Sawyer C.N, McCarty P.L and Parkin G.F (2003). Chemistry for Environmental Engineering and Science (5th ed.). McGraw-Hill Publishers, New York, USA.
Ulrich A and Becker R (2006). Soil parent material is a key determinant of the bacterial community structure in arable soils. FEMS Microbiol Ecol, 56(3):430–443.
Discover more from #1 Microbiology Resource Hub
Subscribe to get the latest posts to your email.


