Mantoux (Tuberculin) Test is used as a laboratory diagnostic aid to detect reactions of individuals to tuberculin i.e. purified protein derivative (PPD). A tuberculin is any preparation that contains tuberculoprotein – which is usually obtained by the filtration of a culture of tubercle bacilli. PPD is the test antigen in the mantoux skin test.
It is obtained by boiling an old broth culture of M. tuberculosis. The filtrate that results from this boiling (known as old tuberculin) is treated with an acid (e.g. trichloroacetic acid) to precipitate tuberculoprotein. The tuberculoprotein is further washed and prepared into standard aqueous solutions known as PPD. In order words, PPD and tuberculin are used interchangeable and may mean the same thing.
Principle: Mantoux test is a skin test for TB in which PPD is injected intradermally on the forearm of the infected TB patient (Figure 1). It measures host’s delayed-type hypersensitivity (DTH) to PPD or tuberculin. The principle of the Mantoux test is based on the development of a cell-mediated immunity (CMI) and a type IV hypersensitivity reaction at the site of injection. Positive Mantoux tuberculin) skin test does not necessarily indicate active TB disease instead it only shows exposure to the pathogen.
Individuals who have been previously immunized against the disease may also respond positively to the skin test but those who have had no contact with Mycobacterium shows no reaction to PPD i.e. they react negatively to the Mantoux test. Only the isolation and identification of tubercle bacilli provides the proof of a TB disease.
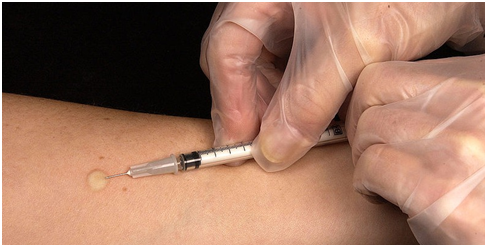
PROCEDURE FOR MANTOUX (TUBERCULIN) TEST
The site of inoculation (i.e. the middle third of the dorsal side of the forearm) is properly disinfected with methylated spirit and allowed to dry before injection. One tuberculin unit (0.1 ml) of PPD is obtained using a 1 ml syringe, and this is injected into the forearm (Figure 1). The site of injection is marked and patients are advised to avoid the temptation of scratching the injection site despite obvious desire to do so as a result of the allergy associated with the test.
The activity of the PPD is expressed in tuberculin units (TU), and the test is interpreted by the area of induration or swelling produced within 48-72 hrs after intradermal injection of tuberculin. The intermediate strength of PPD used in most clinical application of the Mantoux test is 5 TU (an equivalent of 0.000l mg of PPD) but lesser units (e.g. 1 TU) is used initially to avoid unnecessary untoward reactions or higher degree of hypersensitivity in the host’s body.
The lesion formed is measured using a metre rule. Tuberculin test is considered positive if the diameter of the resulting nodule or induration is 10 mm or greater. Absence of an induration or swelling after the specified time shows a negative result. A positive Mantoux test result does not in any way show the activity of the infection but only indicates that the individual may have been previously infected or vaccinated against the disease in the past.
However, prior exposure or infection with other Mycobacteria species and even previous vaccination with the Bacillus Calmette-Guerin (BCG), an attenuated bovine vaccine could result in a false positive test result. Such false positive results should only be deemed suspicious and further analyzed only in cases or places where the BCG vaccine has not been previously used.
The clinical value of the mantoux skin test result (whether negative or positive) depends on the prevalence of reactivity in population and on the incidence of primary TB infection in different age groups. For example, a positive mantoux skin test in young adults, children or infants should raise suspicion of the disease. Also, a negative skin test result especially in regions where the disease has been contained and is uncommon is used as an epidemiological tool to rule out the possibility of a TB disease in the population.
References
Brooks G.F., Butel J.S and Morse S.A (2004). Medical Microbiology, 23rd edition. McGraw Hill Publishers. USA. Pp. 248-260.
Madigan M.T., Martinko J.M., Dunlap P.V and Clark D.P (2009). Brock Biology of microorganisms. 12th edition. Pearson Benjamin Cummings Publishers. USA. Pp.795-796.
Prescott L.M., Harley J.P and Klein D.A (2005). Microbiology. 6th ed. McGraw Hill Publishers, USA. Pp. 296-299.
Ryan K, Ray C.G, Ahmed N, Drew W.L and Plorde J (2010). Sherris Medical Microbiology. Fifth edition. McGraw-Hill Publishers, USA.
Singleton P and Sainsbury D (1995). Dictionary of microbiology and molecular biology, 3rd ed. New York: John Wiley and Sons.
Talaro, Kathleen P (2005). Foundations in Microbiology. 5th edition. McGraw-Hill Companies Inc., New York, USA.
Discover more from #1 Microbiology Resource Hub
Subscribe to get the latest posts to your email.