Erythromycin is a protein synthesis inhibitor that binds to the 50S ribosomal subunit of the bacterial ribosome. It is found in the family of antibiotics known as the macrolides; and antibiotics in this group are generally protein synthesis inhibitors. Other macrolides include azithromycin and clarithromycin. Lincomycin and clindamycin are antibiotics that inhibit protein synthesis in bacteria but are not macrolides. The macrolides specifically block translocation reaction during protein synthesis in bacteria once they bind to the 50S ribosomal subunit.
SOURCES OF ERYTHROMYCIN
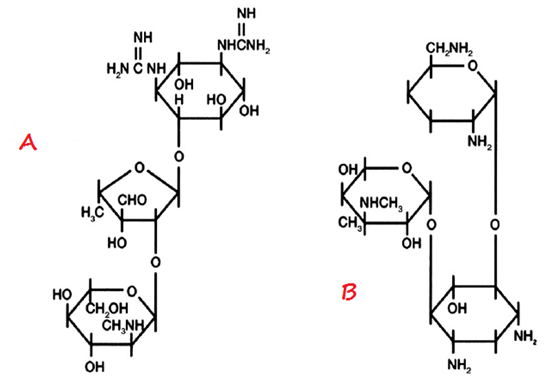
Macrolides are naturally-synthesized by Streptomyces species. Erythromycin is synthesized naturally from a strain of Streptomyces known as S. erythreus. Macrolides can also be semi-synthesized by the chemical modification of their general structure especially by the incorporation of a substituent group into the lactone ring (Figure 1); and this result in the formation of newer macrolides such as azithromycin and clarithromycin.
STRUCTURE OF ERYTHROMYCIN
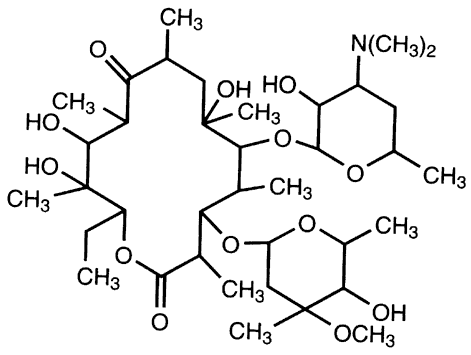
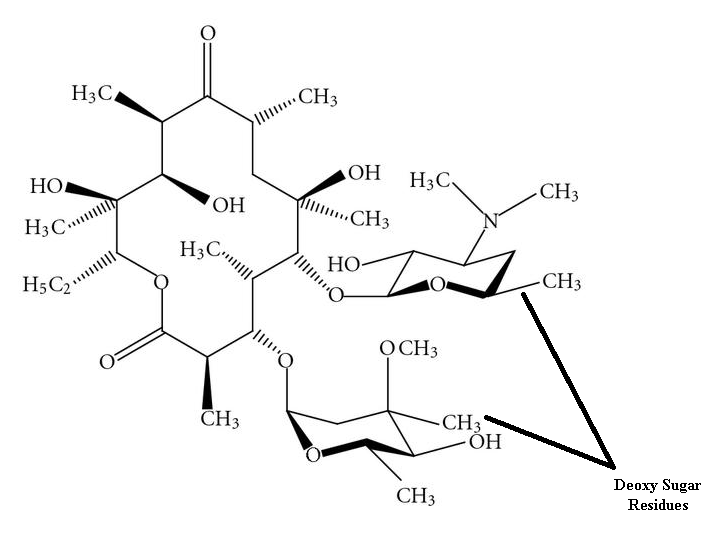
The chemical structure of macrolides (erythromycin) contains large lactone rings that are linked through glycoside bonds with amino sugars. They generally possess a macrocyclic lactone structure (Figure 1). The macrolides structure is usually composed of a large-membered ring that comprises of about 13-15 carbon molecules which are chemically linked to sugar molecules by glycosidic bonds. The chemical structure of erythromycin is shown in Figure 2.
CLINICAL APPLICATION OF ERYTHROMYCIN
Macrolides including erythromycin, clarithromycin and azithromycin are used to treat a wide variety of infections caused by both Gram positive and Gram negative bacteria (Figure 3). Erythromycin is active against the causative agents of whooping cough (caused by Bordetella pertussis), pneumonia (caused by Streptococcus pneumoniae), diphtheria (caused by Corynebacterium diphtheriae), syphilis (caused by Treponema pallidum) and diarrhea (caused by Campylobacter species). They also have activity against mycoplasmas and Chlamydia species. Azithromycin and clarithromycin have broader activity than erythromycin (the first member of the macrolides); and they can also be used to treat some non-bacterial infections such as toxoplasmosis (caused by Toxoplasma gondii) as well as infections caused by mycobacteria.
SPECTRUM OF ACTIVITY OF ERYTHROMYCIN
Erythromycin and the macrolides are generally bacteriostatic in action, and they have broad spectrum of activity since they are active against both pathogenic Gram positive bacteria and Gram negative bacteria. Through generally bacteriostatic in action, macrolides can be bactericidal to some pathogenic Gram positive bacteria.
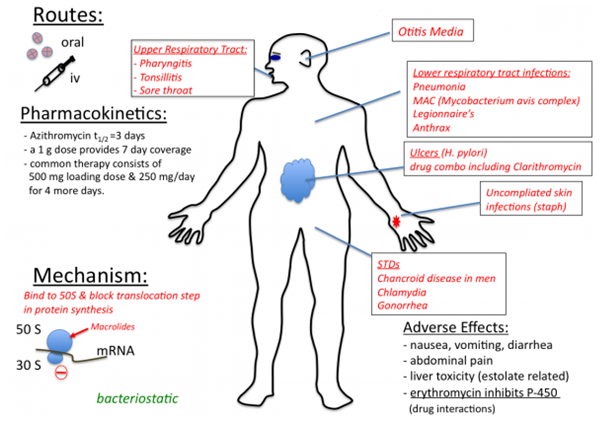
MECHANISM OR MODE OF ACTION
Erythromycin and the other macrolides generally inhibit bacterial protein synthesis by binding to the 50S ribosomal subunit of the bacterial ribosome (particularly the 23S rRNA). Binding of the 50S ribosomal subunit by erythromycin inhibits protein elongation (usually mediated by the enzyme, peptidyl transferase); and this prevents translocation of the ribosome during protein synthesis. Translocation is the movement of some part of the ribosome (especially the mRNA) to other parts of the ribosome (e.g. tRNA) during protein synthesis. Erythromycin blocks the elongation of the polypeptide chain during synthesis when the reversibly bind to the 50S ribosomal subunit, and they are bacteriostatic in action.
BACTERIAL RESISTANCE TO ERYTHROMYCIN
The resistance of pathogenic bacteria to the antibacterial onslaught of the macrolides (including erythromycin) could be due to the influx-efflux mechanism which prevents intracellular accumulation of the drug by actively pumping it out or alteration of the binding site of the antibiotic on the target organism. The alteration of the binding site of erythromycin on the target bacteria is usually mediated by the methylation of the 23S rRNA receptor, which prevents the binding by the antibiotic.
PHARMACOKINETICS OF ERYTHROMYCIN
Erythromycin is easily inactivated by the gastric acid in the GIT, and thus they are poorly absorbed by the body when administered orally. However, the macrolides (inclusive of erythromycin) are distributed widely in the body and they are excreted in urine. It is orally absorbed as stearateor estolate salt, and cerebrospinal fluid (CSF) penetration of erythromycin is poor. It is excreted via biliary and feacal route. Erythromycin is acid labile and the absorption of salt formulations of the drug from the gastrointestinal tract is variable. Thus, erythromycin needs to be administered 2 hrs before or after a meal. The non-salt (base) form of erythromycin is not well absorbed when taken orally, and is used thus for sterilizing the gut before surgery.
SIDE EFFECT/TOXICITY OF ERYTHROMYCIN
The clinical usage of erythromycin and other macrolides is usually associated with some undesirable untoward effects such as mild stomach or GIT upset, vomiting, fever, and diarrhea. Macrolides are generally nontoxic antibacterial agents.
References
Ashutosh Kar (2008). Pharmaceutical Microbiology, 1st edition. New Age International Publishers: New Delhi, India.
Axelsen P. H (2002). Essentials of Antimicrobial Pharmacology. Humana Press, Totowa, NJ.
Balfour H. H (1999). Antiviral drugs. N Engl J Med, 340, 1255–1268.
Bean B (1992). Antiviral therapy: current concepts and practices. Clin Microbiol Rev, 5, 146–182.
Beck R.W (2000). A chronology of microbiology in historical context. Washington, D.C.: ASM Press.
Champoux J.J, Neidhardt F.C, Drew W.L and Plorde J.J (2004). Sherris Medical Microbiology: An Introduction to Infectious Diseases. 4th edition. McGraw Hill Companies Inc, USA.
Chemotherapy of microbial diseases. In: Chabner B.A, Brunton L.L, Knollman B.C, eds. Goodman and Gilman’s The Pharmacological Basis of Therapeutics. 12th ed. New York, McGraw-Hill; 2011.
Chung K.T, Stevens Jr., S.E and Ferris D.H (1995). A chronology of events and pioneers of microbiology. SIM News, 45(1):3–13.
Courvalin P, Leclercq R and Rice L.B (2010). Antibiogram. ESKA Publishing, ASM Press, Canada.
Denyer S.P., Hodges N.A and Gorman S.P (2004). Hugo & Russell’s Pharmaceutical Microbiology. 7th ed. Blackwell Publishing Company, USA. Pp.152-172.
Dictionary of Microbiology and Molecular Biology, 3rd Edition. Paul Singleton and Diana Sainsbury. 2006, John Wiley & Sons Ltd. Canada.
Drusano G.L (2007). Pharmacokinetics and pharmacodynamics of antimicrobials. Clin Infect Dis, 45(suppl):89–95.
Engleberg N.C, DiRita V and Dermody T.S (2007). Schaechter’s Mechanisms of Microbial Disease. 4th ed. Lippincott Williams & Wilkins, Philadelphia, USA.
Finch R.G, Greenwood D, Norrby R and Whitley R (2002). Antibiotic and chemotherapy, 8th edition. Churchill Livingstone, London and Edinburg.
Ghannoum MA, Rice LB (1999). Antifungal agents: Mode of action, mechanisms of resistance, and correlation of these mechanisms with bacterial resistance. Clin Microbiol Rev, 12:501–517.
Gillespie S.H and Bamford K.B (2012). Medical Microbiology and Infection at a glance. 4th edition. Wiley-Blackwell Publishers, UK.
Gordon Y. J, Romanowski E.G and McDermitt A M (2005). A review of antimicrobial peptides and their therapeutic potential as anti-infective drugs. Current Eye Research, 30(7): 505-515.
Hardman JG, Limbird LE, eds. Goodman and Gilman’s The Pharmacological Basis of Therapeutics. 10th ed. New York: McGraw-Hill; 2001.
Joslyn, L. J. (2000). Sterilization by Heat. In S. S. Block (Ed.), Disinfection, Sterilization, and Preservation (5th ed., pp. 695-728). Philadelphia, USA: Lippincott Williams and Wilkins.
Katzung, B. G. (2003). Basic and Clinical Pharmacology (9th ed.). NY, US, Lange.
Kontoyiannis D.P and Lewis R.E (2002). Antifungal drug resistance of pathogenic fungi. Lancet. 359:1135–1144.
Discover more from Microbiology Class
Subscribe to get the latest posts sent to your email.