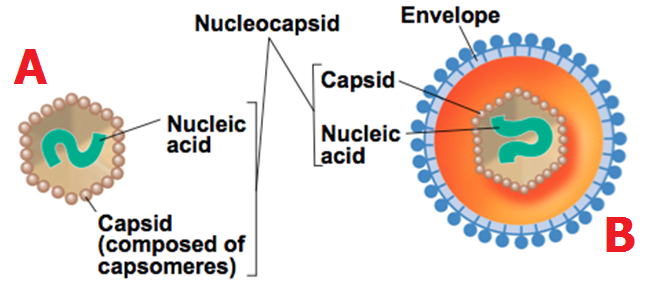
Viruses have several physicochemical properties including pH, molecular size or mass, stability to heat and susceptibility to organic solvents such as ether and other physical or chemical agents that may affect their proper development and replication.
They react to various chemical and physical factors in their environment. These factors inclusive of chemical and physical factors are used to determine the physical and chemical properties of viruses as well as to inactivate them (Table 1).
Table 1. Overview of the physical and chemical properties of viruses
| PHYSICAL PROPERTIES | CHEMICAL PROPERTIES |
| Temperature: Viruses can be inactivated at high temperatures. Several viruses are susceptible to varying degrees of temperature; and this has been successfully used to inactivate viruses for several virological manipulation and even to achieve sterility at a particular time or place. While some viruses can be inactivated at a shorter time (e.g. 30 mins) at a temperature range of 50-60oC, others can be inactivated at the same temperature for a longer time. However, most viruses are inactivated at 100oC at a lesser time interval (e.g. 5-10 mins). | Formaldehyde: Formaldehyde is a chemical that has strong inactivation property on virtually all viruses. Virtually all viruses are susceptible to formaldehyde; and this chemical is usually used as formalin in most disinfectants. Alcohol or ethanol, ethylene diamine and isopropanol amongst other chemicals are examples of other inactivating agents used to deactivate viruses but with low efficacy. Formaldehyde destroys viruses by reacting with viral genomes. |
| Freezing and thawing: The infectivity or degree of virulence of pathogenic viruses can be inactivated when such viruses undergo freezing and thawing; and freezing and thawing are typical physical parameters that generally affect the infectivity and/or antigenic features of viruses. | Ether: Ether is another category of chemical agents that can inactivate viruses. Ether inactivate some classes of viruses especially those with envelopes. Some non-enveloped viruses (i.e. naked viruses) are ether-resistant. The susceptibility of viruses to ether is used to distinguish viruses since naked viruses are mostly ether-resistant while enveloped viruses are ether-sensitive. |
| Radiation: Radiation is generally used to inactivate various viruses. Ultraviolet (UV) light,x-rays, ultrasonicvibrations and sunlightare typical examples of different forms of radiation that can be used to inactivate a virus. However, the inactivation of the virus is largely dependent on the intensity of the radiation used. The higher the intensity of the radiation, the more effective the efficacy of the radiation on the virus. radiation affects the replication processes of viruses. | Chlorine compounds: Chlorine compounds including sodium hypochloride or bleach are used to inactivate viruses. Bleach is a common household disinfectant that is also used in hospitals and industries to disinfect inanimate surfaces. They gave the potential to inactivate viruses by interfering with the replication process of a virus. |
References
Acheson N.H (2011). Fundamentals of Molecular Virology. Second edition. John Wiley and Sons Limited, West Sussex, United Kingdom.
Alan J. Cann (2005). Principles of Molecular Virology. 4th edition. Elsevier Academic Press, Burlington, MA, USA.
Alberts B, Bray D, Johnson A, Lewis J, Raff M, Roberts K and Walter P (1998). Essential Cell Biology: An Introduction to the Molecular Biology of the Cell. Third edition. Garland Publishing Inc., New York.
Barrett J.T (1998). Microbiology and Immunology Concepts. Philadelphia, PA: Lippincott-Raven Publishers. USA.
Black, J.G. (2008). Microbiology: Principles and Explorations (7th ed.). Hoboken, NJ: J. Wiley & Sons.
Brian W.J Mahy and Mark H.C van Regenmortel (2010). Desk Encyclopedia of Human and Medical Virology. Elsevier Academic Press, San Diego, USA.
Brooks G.F., Butel J.S and Morse S.A (2004). Medical Microbiology, 23rd edition. McGraw Hill Publishers. USA.
Cann A.J (2011). Principles of Molecular Virology. Fifth edition. Academic Press, San Diego, United States.
Carter J and Saunders V (2013). Virology: Principles and Applications. Second edition. Wiley-Blackwell, New Jersey, United States.
Champoux J.J, Neidhardt F.C, Drew W.L and Plorde J.J (2004). Sherris Medical Microbiology: An Introduction to Infectious Diseases. 4th edition. McGraw Hill Companies Inc, USA.
Dimmock N (2015). Introduction to Modern Virology. Seventh edition. Wiley-Blackwell, New Jersey, United States.
Dimmock N.J, Easton A.J and Leppard K.N (2001). Introduction to modern virology. 5th edition. Blackwell Science publishers. Oxford, UK.
Discover more from #1 Microbiology Resource Hub
Subscribe to get the latest posts to your email.