Amylase (Starch hydrolysis) test is used to identify bacteria that hydrolyze starch (including amylopectin and amylose) with the help of the enzyme amylase. Amylase is an enzyme that hydrolyzes starch into maltose, glucose, and dextrin’s.
Some bacterial isolates including Bacillus and Clostridium have the ability to produce α-amylase and other enzymes that degrade starch molecules into smaller and simple sugars (e.g. glucose), and this serves as the basis for the identification of such microorganisms in the laboratory.
PROCEDURE FOR AMYLASE (STARCH HYDROLYSIS) TEST
- Perform this test using pure cultures of the test isolate.
- Prepare nutrient agar plates according to manufacturer’s instructions.
- Pour thin layers of the prepared agar on Petri dish plate(s) and allow to set.
- Prepare another smaller quantity of nutrient agar base modified with 1 % soluble starch and sterilize by autoclaving. This is the starch agar medium.
- Pour the starch agar medium on the already poured thin layer of nutrient agar earlier prepared to make a very light upper layer. In some cases, iodine is incorporated in the agar medium during preparation.
- Allow plate(s) to set and cool.
- Inoculate the test bacteria by a single streak or inoculate isolate at the center of the Petri dish.
- Incubate plates at 37oC for 18-24 hrs.
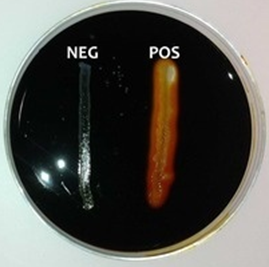
Flood plate(s) with iodine and drain off excess iodine leftovers. Iodine is known to react with starch to give a blue-black color. Observe the plate(s) for a change in colouration. Iodine reacts with the starch in the media to form a dark-brown or yellow-halo colour that results in a clear zone around the bacterial growth on the agar plate. This is indicative of a positive starch hydrolysis test result. Absence of this colouration shows a negative result (Figure 1).
References
Basic laboratory procedures in clinical bacteriology. World Health Organization (WHO), 1991. Available from WHO publications, 1211 Geneva, 27-Switzerland.
Beers M.H., Porter R.S., Jones T.V., Kaplan J.L and Berkwits M (2006). The Merck Manual of Diagnosis and Therapy. Eighteenth edition. Merck & Co., Inc, USA.
Biosafety in Microbiological and Biomedical Laboratories. 5th edition. U.S Department of Health and Human Services. Public Health Service. Center for Disease Control and Prevention. National Institute of Health. HHS Publication No. (CDC) 21-1112.2009.
Cheesbrough M (2010). District Laboratory Practice in Tropical Countries. Part I. 2nd edition. Cambridge University Press, UK.
Cheesbrough M (2010). District Laboratory Practice in Tropical Countries. Part 2. 2nd edition. Cambridge University Press, UK.
Collins C.H, Lyne P.M, Grange J.M and Falkinham J.O (2004). Collins and Lyne’s Microbiological Methods. Eight edition. Arnold publishers, New York, USA.
Disinfection and Sterilization. (1993). Laboratory Biosafety Manual (2nd ed., pp. 60-70). Geneva: WHO.
Garcia L.S (2010). Clinical Microbiology Procedures Handbook. Third edition. American Society of Microbiology Press, USA.
Garcia L.S (2014). Clinical Laboratory Management. First edition. American Society of Microbiology Press, USA.
Fleming, D. O., Richardson, J. H., Tulis, J. I. and Vesley, D. (eds) (1995). Laboratory Safety: Principles and practice. Washington DC: ASM press.
Dubey, R. C. and Maheshwari, D. K. (2004). Practical Microbiology. S.Chand and Company LTD, New Delhi, India.
Gillespie S.H and Bamford K.B (2012). Medical Microbiology and Infection at a glance. 4th edition. Wiley-Blackwell Publishers, UK.
Discover more from #1 Microbiology Resource Hub
Subscribe to get the latest posts to your email.



