Antigen-antibody reaction is an immunological reaction in which a particular antibody molecule reacts with a specific antigen to form an antigen-antibody complex which is marked for further immunological response by other components of the immune system. It can occur inside a living organism (i.e. in vivo reaction) and it can also occur outside the host body (i.e. in vitro reaction).
The main biological function of an antigen-antibody reaction within the body of the host organism (i.e. in vivo) is to identify and mark antigens or pathogenic microorganisms for destruction and elimination from the body by other specific components of the immune system e.g. pathogen destruction by phagocytes.
Antigen-antibody reaction is a reversible immunological reaction; and though it bears a resemblance to the notable enzyme-substrate reaction, there is no apparent degradation in the chemical structure of either the antibody or antigen involved in the reaction.
Immunoglobulins generally have a “Y” shaped structure that comprises of two main parts which is the antigen-binding fragment (Fab) and the crystallizable fragment (FC) which binds antigens and host cells respectively. Antibodies generally react with antigens by binding the antigens specifically at their epitopes or antigenic determinant sites using their antigen-binding fragments (Figure 1).
The complementarity determining regions (CDRs) of the antibody react with the epitopes or antigenic determining sites of the antigen(s) to form an immune complex known as antigen-antibody complex which is held together by non-covalent bonds or forces such as van der Waals forces and hydrogen bonds amongst others.
CDRs which can also be called hyper-variable regions (HVRs) are specific areas within the variable regions of an immunoglobulin molecule (i.e. the VH/VL domain) as well as the TCRs of T cells that specifically bind to antigens, and thus determine the antigen-binding specificity of the molecule.
The strength of antigen-antibody binding or interaction is generally known as avidity. Avidity is defined as the functional binding strength between an antigen and antibody; and it generally reflects the interaction between the epitopes and the CDRs/HVRs of the interacting immunoglobulin molecule(s).
Avidity expresses the binding power or capacity of an immunoglobulin molecule rather than its affinity i.e. its attraction to particular epitopes of an antigenic molecule. It is the ability of an immunoglobulin molecule to express multiple interactions (especially with multivalent antibodies such as IgM) with an antigen.
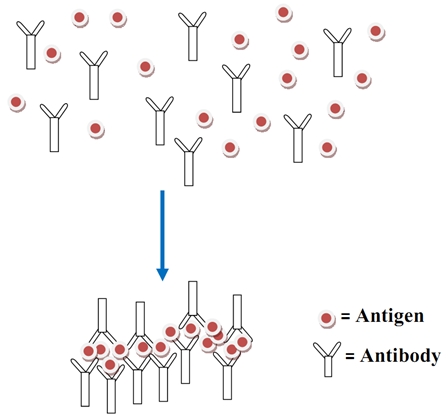
Immunoglobulins react specifically with the surfaces of pathogenic microorganisms or soluble antigens (that invade the body) in an immunological fashion; and this interaction between the antigens and the antibodies leads to the formation of an antigen-antibody complex as earlier stated. The formation of the antigen-antibody complex produces an observable clump (especially in vitro)to form agglutinates and precipitates in agglutination and precipitation reaction respectively.
Precipitation reaction is an immunological reaction in which an antigen reacts with antibodies known generally as precipitins to form visible clumps or precipitates. Antigen-antibody reaction whether in vivo or in vitro is of tremendous clinical or medical importance because the formation of antigen-antibody complexes in vivo for example makes pathogenic particles or soluble antigens to be easily opsonized and phagocytosed by phagocytes, macrophages and other components of the immune system.
The immune system is stimulated to respond immediately and mount an immunological response that will result to the elimination of the invading disease agent or foreign body from the host body. In addition, the formation of antigen-antibody complexes in vivo also makes pathogenic microorganisms to be easily neutralized by other components of the immune system.
For in vitro or reaction, antigen-antibody complexes that result in the formation of visible clumps known as agglutinates in agglutination reaction or precipitates in precipitation reaction help medical scientists to unravel the causative agents of some infectious diseases; and this techniques have been widely used in some rapid diagnostic tests (otherwise known as agglutination tests for example) to detect the presence of antibodies in patients serum or plasma after an infection or disease process in the host.
Typical examples of in vivo antigen antibody reaction include ADCC, toxin neutralization, inflammation, the complement system and neutralization of toxins amongst others; and in vivo antigen-antibody reaction is mainly aimed at protecting the host from the negative consequences of antigens or pathogenic microorganisms that penetrate the body.
Agglutination is simply defined as the visible clumping of particulate antigens (e.g. bacteria) by an antibody (otherwise known as agglutinins). Agglutination reaction can also occur with erythrocytes or red blood cells (RBCs); and this type of agglutination is generally known as haemagglutination reaction. Haemagglutination reaction is caused by haemagglutinins just as agglutinins and precipitins cause agglutination and precipitation reactions respectively.
Agglutinins are antibodies that cause visible clumping of particulate antigens. They are generally immunoglobulin molecules that produce such immunological reactions (i.e. agglutination) in which visible clumps are formed when antibodies react specifically with antigens.
Such interaction result in the formation of visible clumps; and this principle of agglutination reaction is applied in microbiology laboratory (particularly in serology) to determine the presence of antigens or antibodies in patient’s clinical specimens (e.g. serum or plasma). The Widal test used for the laboratory diagnosis of Salmonella infection is based on the principle of agglutination test.
Precipitation reaction is the immunological interaction of a soluble antigen with a soluble immunoglobulin molecule (precipitin) to form an insoluble complex.Precipitation test is used to detect streptococcal group antigens. It is noteworthy that the reaction between an antibody and an antigen is highly specific in nature; and because of this specificity the notable interactions between immunoglobulin molecules and antigenic molecules have been exploited by medical scientists to determine the presence of either an antigen or antibody in clinical samples in the course of unraveling the causative agents of some infectious diseases.
This is the basis for most serological tests carried out in the microbiology or serology laboratories of hospitals.Nevertheless, cross-reactivity can occur in some cases between an antigen and unrelated immunoglobulin molecule and vice-versa; and cross-reactivity usually occurs in scenarios where two antigenic molecules share corresponding epitopes, a biological phenomenon that stimulate an antibody to bind or interact with an unrelated antigenic molecule.
Cross-reactivity is the capacity of a given immunoglobulin molecule or T cell receptor (TCR) to react with multiple antigens that share common antigenic determinants or epitopes. A typical example of cross-reactivity is observed in ABO blood group antigens that possess polysaccharide antigens that contain similar oligosaccharide residues; and the cross-reactivity exhibited by ABO blood groups in humans has played significant roles in blood typing and even in transplantation or tissue/organ grafting.
Agglutination reaction can also be employed in the identification of bacterial pathogens (e.g. Salmonella, Shigella, and Streptococcus species) in the microbiology laboratory. In the typing of ABO blood group of humans (i.e. in haemagglutination reaction), a drop of blood from an individual is mixed with antiserum antigens (e.g. antiserum A or B) on a slide; and the formation of visible clumps by the test blood sample with any of the corresponding antiserum antigen confirms the individuals blood group.
For example, if the person’s blood sample formed visible clumps with antiserum A, then the person’s blood group is blood group A. The specificity between antigens and antibodies as earlier said are used in a variety of quantitative or qualitative immunologic assays to diagnose the causative agent(s) of infectious diseases.
REFERENCES
Abbas A.K, Lichtman A.H and Pillai S (2010). Cellular and Molecular Immunology. Sixth edition. Saunders Elsevier Inc, USA.
Actor J (2014). Introductory Immunology. First edition. Academic Press, USA.
Alberts B, Bray D, Johnson A, Lewis J, Raff M, Roberts K and Walter P (1998). Essential Cell Biology: An Introduction to the Molecular Biology of the Cell. Third edition. Garland Publishing Inc., New York.
Bach F and Sachs D (1987). Transplantation immunology. N. Engl. J. Med. 317(8):402-409.
Barrett J.T (1998). Microbiology and Immunology Concepts. Philadelphia, PA: Lippincott-Raven Publishers. USA.
Jaypal V (2007). Fundamentals of Medical Immunology. First edition. Jaypee Brothers Medical Publishers (P) Ltd, New Delhi, India.
John T.J and Samuel R (2000). Herd Immunity and Herd Effect: New Insights and Definitions. European Journal of Epidemiology, 16:601-606.
Levinson W (2010). Review of Medical Microbiology and Immunology. Twelfth edition. The McGraw-Hill Companies, USA.
Roitt I, Brostoff J and Male D (2001). Immunology. Sixth edition. Harcourt Publishers Limited, Spain.
Zon LI (1995). Developmental biology of hematopoiesis. Blood, 86(8): 2876–91.
Discover more from #1 Microbiology Resource Hub
Subscribe to get the latest posts to your email.


