GMP encompasses the rules governing the manufacture of a safe and efficacious pharmaceutical product, drug […]
Tag: good manufacturing practice
GOOD MANUFACTURING PRACTICE (GMP)
Good manufacturing practice (GMP) is simply defined as those general rules that govern the manufacture […]
Quality Assurance: System Design and Implementation in Pharmaceutical Manufacturing and Clinical Laboratory Practice
Quality Assurance (QA) is a structured, systematic, and documented framework designed to ensure that products […]
Quality Control in Pharmaceutical Manufacturing: Principles, Microbial Testing, Practical Implementation & GMP Compliance
Introduction Quality control (QC) is a systematic monitoring and verification framework used to detect, measure, […]
PYROGEN TEST
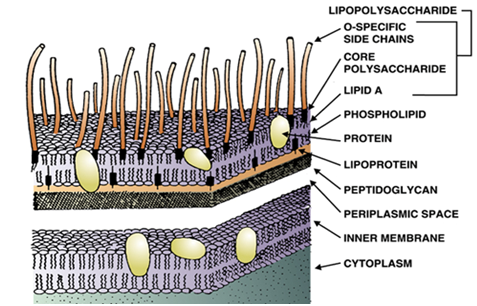
A pyrogen is simply defined as a fever-causing (inducing) agent that includes toxins of microorganisms. […]