There are different types of steps involved in the transformation of a bacterial cell in gene cloning techniques. Bacterial cells are transformed when they are infused with exogenous DNA from another organism, and this makes them competent enough to express the gene product (or protein) of the gene they are carrying. Usually, the exogenous DNA from the donor cell which is later introduced into the recipient cell by transformation techniques is usually the gene of interest, and such genetic particle or DNA contains specific gene sequences with immense economic and health benefit and which the researcher seeks to multiply in the recipient bacterial cell. The resultant cells generated are clones of the original cells because they are derived from a single cell; and such clones usually contain copies of the same recombinant plasmid with its fragment and exogenous DNA molecule.
Transformation is the genetic process of transferring exogenous DNA from a donor cell into a recipient cell. A bacterial cell can be transformed in a variety of ways such as by chemical means (e.g. use of calcium chloride) and by physical methods (e.g. electroporation). Exogenous DNA carrying the specific gene of interest can be introduced into recipient cells through chemical method using calcium chloride and electroporation which involves the use of an electric force or charge to facilitate the DNA uptake by the recipient cell. Heat shock is also applied in the chemical methods using heat-shock processes that promote the entry of the exogenous DNA into the bacterial cell. Transformation is usually the finally step in any cloning technique; and it precedes the selection and expression of the cloned gene.
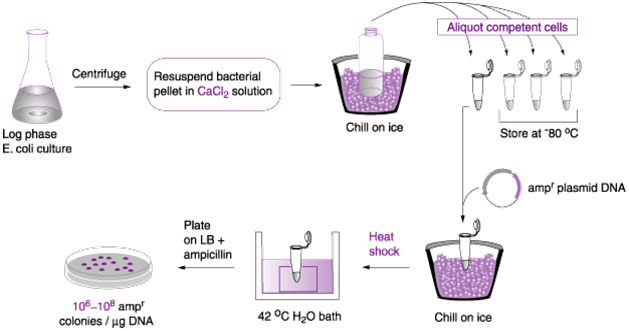
- Transformation with calcium chloride: Calcium chloride (CaCl2) solution is usually used to chemically make bacterial cells competent enough to take up exogenous DNA molecule. In this method of transformation, the recipient bacterial cells (e.g. E. coli) is first grown in a growth medium and harvested at its exponential phase. And the cells are mixed with CaCl2 in a suspension that also contains the plasmid vector, and this occurs in a flask or tube (Figure 1).
The tube containing the suspension of the bacterial cell, CaCl2 solution and the plasmid vector is centrifuged to ensure even mixture of the solution; and it is chilled in ice and passed through a heat-shock process (at 42oC for 60 seconds) that make the bacterial cell to be more adaptable to the exogenous DNA molecule. The heat shock causes damage to the cell wall of the bacterium, and this allows the foreign DNA to penetrate the recipient bacterial cell.
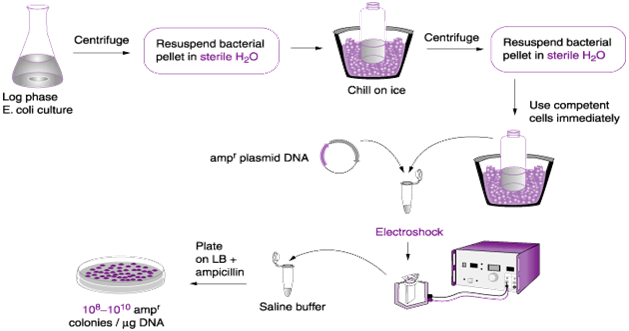
- Transformation by electroporation: Electroporation is the in vitro molecular biology technique that is used to increase the efficiency of transforming a bacterial cell. It is a physical method of transforming bacterial cells using electric charges or pulses (Figure 2). In electroporation, bacterial cells to be transformed are grown in a growth media and then harvested at their log or exponential growth phase when their development is still active.
The harvested bacterial cell is mixed in a buffer inside a tube or cuvette, and the cuvette is then place in an electrical vessel that passes electrical charges through it. The high voltage pulses that passes through the bacterial cell in the tube makes the cell wall of the organism to be more permeable to exogenous DNA which it takes up more quickly than before; and electroporation can be used for a wide variety of other living system such as plant cells.
- Transformation using viral agents: Bacterial cells can be transformed using some viral particles including adenoviruses and retroviruses. This process of transforming cells using a virion is generally known as transduction. Transduction is a virus-mediated transfer of exogenous DNA from a donor cell to a recipient cell. In transduction viruses or bacteriophages act as vehicles or vectors that carry the gene of interest into the target host cell.
Transfection refers to the in vitro techniques in which exogenous DNA molecule is introduced into a recipient host cell in order to genetically modify the latter. Transfection is also the process of introducing foreign DNA into cells (especially eukaryotic cells) using non-viral vectors. It is an efficient method of delivering the gene of interest into the recipient host cell; and transfection is applied in a variety of molecular biology techniques to determine gene function and gene expression in organisms.
- Transformation by other techniques: Aside transformation by electroporation and CaCl2 which are both physical and chemical means of transforming bacterial cells respectively, the recipient bacterial cell can also be transformed by other molecular techniques which encourage or promote the entry of the exogenous DNA into the target bacterial cell. Some of these other transformation techniques include lipofection and microinjection amongst others.
Transformation is generally an inefficient process of making bacterial cells competent enough to uptake exogenous DNA molecules. But the process can be improved upon since the plasmid DNA used usually has selectable markers (e.g. antibiotic resistance gene) which help in their easy detection and isolation after transformation; and this prevent the possibility of having to screen all the colonies on the plate for transformed bacterial cells.
Thus, it is easy to screen and isolate those bacterial cells that have taken up the foreign DNA as they are the only once that will grow on the agar medium which contain antibiotics that inhibit the growth of non-competent and untransformed bacterial cells.
The choice of microorganisms for many recombinant DNA technology processes and gene cloning techniques in which DNA molecules are deliberately manipulated is usually bacteria and fungi especially Escherichia coli and the yeast cell, Saccharomyces cerevisiae because these organisms have been well characterized and used over periods of time for such molecular experimentations.
References
Alberts B, Bray D, Lewis J, Raff M, Roberts K and Watson J.D (2002). The molecular Biology of the Cell. Fourth edition. New York, Garland, USA.
Chen I and Dubnau D (2004). DNA uptake during bacterial transformation. Nat. Rev. Microbiol. 2 (3): 241–249.
Cooper G.M and Hausman R.E (2004). The cell: A Molecular Approach. Third edition. ASM Press.
Dale J (2003). Molecular genetics of bacteria. Jeremy W. Dale and Simon Park (4th eds.). John Wiley & Sons Ltd, West Sussex, UK. Pp. 312-313.
Das H.K (2010). Textbook of Biotechnology. Fourth edition. Wiley edition. Wiley India Pvt, Ltd, New Delhi, India.
Lewis R (2004). Human Genetics: Concepts and Applications. Sixth edition. McGraw Hill Publishers, USA.
Lodish H, Berk A, Matsudaira P, Kaiser C.A, Kreiger M, Scott M.P, Zipursky S.L and Darnell J (2004). Molecular Cell Biology. Fifth edition. Scientific American Books, Freeman, New York, USA.
Madigan M.T., Martinko J.M., Dunlap P.V and Clark D.P (2009). Brock Biology of Microorganisms, 12th edition. Pearson Benjamin Cummings Inc, USA.
McPherson M and Moller S (2002). PCR: The Basics. 2nd edition. Taylor and Francis Group. New York, USA.
Sambrook, J., Russell, D.W. (2001). Molecular Cloning: a Laboratory Manual, 3rd edn. Cold Spring Harbor Laboratory Press, New York.
Synder L, Peters J.E, Henkin T.M and Champness W (2013). Molecular Genetics of Bacteria. Fourth edition. American Society of Microbiology Press, USA.
Tamarin Robert H (2002). Principles of Genetics. Seventh edition. Tata McGraw-Hill Publishing Co Ltd, Delhi.
Discover more from Microbiology Class
Subscribe to get the latest posts sent to your email.