Proteins are a group of polypeptides that form a molecule of specific biological function; and they comprises mainly of amino acid sequences that are held together by a special type of covalent bonds known as peptide bonds. They are macromolecules that consist of long chains of amino acids held together in structural forms known as polypeptides. Other macromolecules include nucleic acids (i.e. DNA and RNA), lipids (i.e. fats and oils) and carbohydrates. DNA and RNA are informational macromolecules because they store, transport and store genetic information.
Various types or classifications of proteins exist; and this includes but not limited to: catalytic proteins which are usually enzymes that drive or accelerate the rate of chemical reactions in the cell; regulatory proteins e.g. hormones which act as chemical messengers within the cell; transport proteins e.g. haemoglobin found in red blood cells, and which transport O2 from one location to another in animals; and structural proteins e.g. keratin and melanin which both play several biological roles in the skin of higher animals amongst others.
Enzymes are the catalysts for chemical reactions in the cell of an organism while structural proteins forms the integral parts of the major structures or components of a cell such as the cell membranes amongst others. Protein synthesis is usually the final step in the expression of gene in any living organisms; and they play several important roles in the cell. While both the DNA and RNA are chiefly responsible for the storage, transfer and processing of genetic information (as contained in the genome), the proteins form the main cellular machinery in which the genetic program of the DNA or gene is expressed.
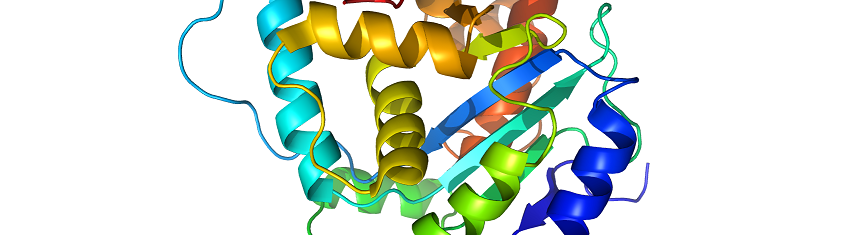
Thus, it is at the protein level that gene function is actually expressed; and the biological function of a protein molecule is mainly determined by the tertiary or three-dimensional (3-D) structure of that particular protein. Proteins are therefore the main gene product of the cell. These important informational macromolecules are synthesized in the ribosomes of the cell; and they assume various structures.
Proteins are generally known to exist in four (4) morphological forms which are:
- Primary structure,
- Secondary structure,
- Tertiary structure and
- Quaternary structure.
PRIMARY STRUCTURE
The primary structure of a protein molecule is the protein’s amino acid sequence; and it can also be called the polypeptide or polypeptide chain. A polypeptide is a chain of amino acid sequence held together by a type of covalent bond known as peptide bond (-CO.NH-). The polypeptides are folded into a three dimensional structure which determines the biological role of a protein molecule in the cell of an organism.
The primary structure of a protein is the informational macromolecule such as a polypeptide which is the main building blocks of proteins. It is the precise sequence of the monomeric units that make a protein molecule. In other words, primary structure is the specific sequence of amino acids in a polypeptide chain. Proteins are polymers of amino acids covalently bonded together by peptide bonds.
The linking of amino acids through peptide bonds leads to the formation of polypeptides, which are polymers of amino acids bonded to one another by peptide bonds. Thus the linear array of amino acids in a polypeptide is called the primary structure of the protein. Proteins consist of one or more polypeptides; and protein molecules differ in their number of amino acids i.e. the number of amino acid present in proteins differs from one protein molecule to another protein molecule.
Therefore, the primary structure of a polypeptide is important to the final function of the protein molecule because it is consistent with only certain types of folding patterns. Only the final, folded polypeptide takes up a particular biological activity in the cell of the organism. It is noteworthy that the primary structure of each protein molecule is determined by the sequence of specific amino acids as encoded by the messenger RNA (mRNA), which processes and directs the proper folding of the polypeptide chain into the secondary structure. The primary structure serves as the foundational basis from which other protein structures including the secondary, tertiary and quaternary structures are formed.
SECONDARY STRUCTURE
The secondary structure of a protein molecule is the local folding of a polypeptide. It is the initial pattern of folding of a polypeptide which is usually dictated by opportunities for hydrogen bond. The alpha (α) helix is a typical example of a secondary structure. The alpha helix is a region of the polypeptide that folds into a corkscrew shape. It is not worthy that a polypeptide does not continue to remain a linear structure after been formed.
The linear structure so formed (especially in the primary structure-state) has to transform into a different morphological form, in this case the helical structure that depicts the secondary structure of a protein molecule. Transformation of a linear protein structure into folds (i.e. the alpha-helix) gives it a more stable organization or structural pattern. Linear structures of a polypeptide twist and fold in specific patterns to form the secondary structure.
The α-helix is mainly joined together by hydrogen bonds which gives the molecule its stability. In some cases, a different type of secondary structure known as the beta (β) sheet is formed from the polypeptide chain. In the β-sheet structure, chains of amino acids in the polypeptide folds back and forth upon itself; and this phenomenon results in the formation of a β-sheet secondary structure instead of the alpha helix. Generally, secondary structure is the folding of the polypeptide chain into specific shapes such as the alpha helix and beta sheets amongst others.
TERTIARY STRUCTURE
The tertiary structure of a protein molecule is the final folded structure of a polypeptide that has previously attained a secondary structure. It is the additional folding of a protein molecule into its characteristic shape. Other regions of a secondary structure may include helixes, strands, coils, turns and random coils that usually interact chemically with each other to form the unique three-dimensional shape of the protein molecule known as the tertiary structure.
Once a polypeptide has achieved secondary structure it continues to fold to from an even more stable molecule which finally results in the formation of the unique three-dimensional molecule characteristic of the tertiary structure of a protein molecule.
The three-dimensional structure of protein is important because it defines the shape and size of the protein as well as its biological activity or function in the cell. A correct three dimensional shape is essential for the chemical or physical activity of a protein within the cell of an organism.
QUATERNARY STRUCTURE
Quaternary structure is the number and types of individual polypeptides in the final protein molecule. It is the type of protein molecule formed when two or more protein molecules are covalently held together. For some proteins, a single polypeptide chain folded in its proper three-dimensional structure creates the final protein.
But this is not always the case as many proteins have several different polypeptide subunits that make the final active protein. For these protein molecules, the chemical interactions between the different subunits form the quaternary structure.
FUNCTIONS OF PROTEINS IN THE CELL
- Proteins are the instruments through which the genetic potential of an organism are expressed since genetic information flows from the DNA to the RNA and then to protein.
- They play vital roles in almost all cellular and metabolic processes of a cell. For example, structural proteins and regulatory proteins maintain the structural integrity of the cell as well as direct the metabolic activities as chemical messengers respectively.
- Enzymes, which are catalytic proteins, act as catalysts to promote several chemical and biological reactions in the cell. Enzymes are regulatory proteins that accelerate the rate of reaction in the cell.
- Proteins constitute the components that make up the organelles and organs of unicellular and multicellular organisms respectively.
- Haemoglobin is a protein found in the cytoplasm of red blood cells (RBCs), and they give blood their characteristic red colour as well as help in the transportation of oxygen molecules from one cell or part of the body to another. Haemoglobin is a typical example of transport proteins.
- Structural proteins such as keratin and melanin found in the skin cells as well as proteins that make up the muscles and skeletal structure of higher organisms or animals assist in maintaining the structural integrity of the skin and they also aid in their movement.
References
Alberts B, Bray D, Johnson A, Lewis J, Raff M, Roberts K andWalter P (1998). Essential Cell Biology: An Introduction to the Molecular Biology of the Cell. Third edition. Garland Publishing Inc., New York.
Ausubel, F.M., Brent, R., Kingston, R.E., Moore, D.D., Seidman, J.G., Smith, J.A., Struhl, K., eds (2002). Short Protocols in Molecular Biology, 5th edn. John Wiley & Sons, New York.
Bains W (1998). Biotechnology: From A to Z. 2nd ed. Oxford University Press, New York, USA.
Branden C and Tooze J (1998). Introduction to protein structure. A non-technical introduction to protein structure. New York: Garland Press.
Chen I and Dubnau D (2004). DNA uptake during bacterial transformation. Nat. Rev. Microbiol. 2 (3): 241–249.
Cooper G.M and Hausman R.E (2004). The cell: A Molecular Approach. Third edition. ASM Press.
Dale J (2003). Molecular genetics of bacteria. Jeremy W. Dale and Simon Park (4th eds.). John Wiley & Sons Ltd, West Sussex, UK. Pp. 312-313.
Dale J (2003). Molecular genetics of bacteria. Jeremy W. Dale and Simon Park (4th eds.). John Wiley & Sons Ltd, West Sussex, UK. Pp.
Glick B.R and Pasternak J.J (2003). Molecular Biotechnology: Principles and Applications of Recombinant DNA. ASM Press, Washington DC, USA.
Hames B.D and Rickwood D (1998). Gel Electrophoresis of Proteins: A Practical Approach 3rd Edition. The Practical Approach Series, Oxford University Press
Latha C.D.S and Rao D.B (2007). Microbial Biotechnology. First edition. Discovery Publishing House (DPH), Darya Ganj, New Delhi, India.
Lewis R (2007). Human Genetics: Concepts and Applications. Seventh edition. McGraw-Hill Companies, Inc, New York, USA.
Lodish H, Berk A, Matsudaira P, Kaiser C.A, Kreiger M, Scott M.P, Zipursky S.L and Darnell J (2004). Molecular Cell Biology. Fifth edition. Scientific American Books, Freeman, New York, USA.
Madigan M.T., Martinko J.M., Dunlap P.V and Clark D.P (2009). Brock Biology of Microorganisms, 12th edition. Pearson Benjamin Cummings Inc, USA.
Tamarin Robert H (2002). Principles of Genetics. Seventh edition. Tata McGraw-Hill Publishing Co Ltd, Delhi.
Thieman W.J, Palladamo M.A and Thieman W (2003). Introduction to Biotechnology. Benjamin Cummings, San Francisco, CA.
Discover more from Microbiology Class
Subscribe to get the latest posts sent to your email.