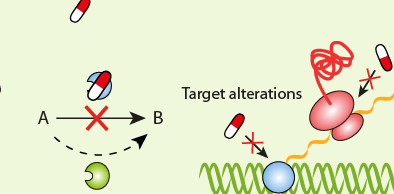
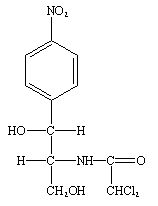
GMP guidelines are not prescriptive instructions on how to manufacture any product including food, drugs, and other pharmaceutical products. Rather, the GMPs are a series of general principles that must be observed during the manufacturing of pharmaceuticals, drugs and food in order to ensure that only products of high quality and one which is free from contamination are produced. It is the company’s responsibility to determine the most effective and efficient quality process for manufacturing; and it is critical that they set their GMP guidelines to meet these quality requirements for manufacturing. Though the good manufacturing guidelines may vary from company to company or from place to place, all the guidelines usually follow a basic set of principles as follows:
- Manufacturing processes are clearly defined and controlled. All critical processes are validated to ensure consistency and compliance with specifications.
- Manufacturing processes are controlled, and any changes to the process are evaluated. Changes that have an impact on the quality of the drug are validated as necessary.
- Instructions and procedures for manufacturing are written in clear and unambiguous language that anyone can understand.
- Operators in pharmaceutical and food manufacturing companies are trained to carry out and document procedures at every stage of the manufacturing processes.
- Records are made, manually or by instruments, during manufacture that demonstrate that all the steps required by the defined procedures and instructions were in fact taken and that the quantity and quality of the drug was as expected. Deviations are investigated and documented.
- Records of manufacture (including distribution) that enable the complete history of a batch of product to be traced are retained in a comprehensible and accessible form.
- The distribution of the drugs minimizes any risk to their quality.
- A system should be available for recalling any batch of drug or product from sale or supply especially for products suspected to be sub-standard, faulty or adulterated.
- Complaints about marketed drugs are examined, the causes of quality defects are investigated, and appropriate measures are taken with respect to the defective drugs and to prevent recurrence of such problem in subsequent productions.
References
Arora D.R (2004). Quality assurance in microbiology. Indian J Med Microbiol, 22:81-86.
Ashutosh Kar (2008). Pharmaceutical Microbiology, 1st edition. New Age International Publishers: New Delhi, India.
Axelsen P.H (2002). Essentials of antimicrobial pharmacology. Humana Press, Totowa, New Jersey, USA. Al-Jasser A.M (2006). Extended – Spectrum Beta – Lactamases (ESBLs): A Global Problem. Kuwait Medical Journal, 38(3):171-185.
Bisht R., Katiyar A., Singh R and Mittal P (2009). Antibiotic Resistance – A Global Issue of Concern. Asian Journal of Pharmaceutical and Clinical Research, 2 (2):34-39.
Block S.S (2001). Disinfection, sterilization and preservation. 5th edition. Lippincott Williams & Wilkins, Philadelphia and London.
Nally J.D (Ed.) (2007). Good manufacturing practices for pharmaceuticals. Sixth edition. Informa Healthcare USA, Inc, New York.
Discover more from Microbiology Class
Subscribe to get the latest posts sent to your email.