Fusidic acid is a bacteriostatic antimicrobial agent and/or antibiotic that is derived from the fungus, Fusidium coccineum. It is used as a topical antimicrobial agent to treat skin infections caused by pathogenic microbes including Staphylococcus aureus and Propionibacterium acne. Fusidic acid is an antibiotic that belongs to a group of antimicrobials known as the fusidanes. The molecule has a steroid-like structure but does not possess any steroid activity.
The anti-microbial activity of fusidic acid is specifically aimed at the most common skin pathogens, including Staphylococcus aureus, towards which it is one of the most potent antibiotics. Fusidic acid has great application in dermatology. Dermatology is the branch of medicine that deals with the diseases of the skin, nails and hair.
The place of fusidic acid in dermatology is in the treatment of mild to moderately severe skin infections and soft-tissue infections including but not limited to: impetigo, folicullitis, erythrasma, furunculosis, abscesses and infected traumatic wounds. Fusidic acid is used to treat acne (caused by Propionibacterium acne).However, fusidic acid is of less use in other similar health conditions such as hidradenitis suppurative (also known as acne inversa), chronic leg ulcers, burns and pressure sores.
Hidradenitis suppurative is a chronic skin disease characterized by clusters of abscesses or subcutaneous boil-like infections that commonly affects the underarms, under the breasts, inner thighs, groin, and the buttocks. Fusidic acid is a bacteriostatic antibiotic that is often used topically in creams and eye drops as antimicrobial agents. However, fusidic acid can also be given systemically as tablets or injections to sick patients.
The main forms in which fusidic acid can be used for medical therapy include: parenteral usage, topical usage and oral usage. Fusidic acid is often used clinically in its sodium salt form known as sodium fusidate. Sodium fusidate is the main active ingredient of fusidic acid. Other chemical names for fusidic acidinclude:Fusidine, Ramycin, Fucithalmic and Fucidin acid. The molecular formular of fusidic acid is: C31H48O6. The structure of fusidic acid is shown in Figure 1.
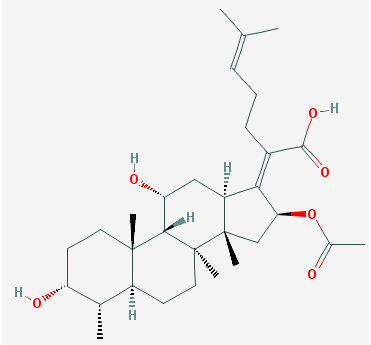
Source: Fusidic acid is derived naturally from the fungus Fusidium coccineum. It is mainly used as a topical medication to treat skin infections. It can also be naturally sources from Mucor ramannianus.
Mode of action: Fusidic acid is a bacterial protein synthesis inhibitor and it is bacteriostatic in action because it does not kill the invading bacteria, but it only inhibits bacterial protein synthesis by blocking translation. Fusidic acid acts as a bacterial protein synthesis inhibitor by preventing the turnover of elongation factor G (EF-G) from the ribosome. The prevention of the turnover of EF-G causes the interruption of the peptide-chain elongation – which blocks the A site of ribosomes.
This action leads to the misreading of the genetic code for protein synthesis. And this in turn inhibits the process of translation in the organism, thereby preventing protein formation or synthesis. Fusidic acid is effective primarily on Gram-positive bacteria including Staphylococcus species, Streptococcus species, most coagulase-positive staphylococci, Clostridium species, methicillin resistant Staphylococcus aureus (MRSA) strains and Corynebacterium species. It has no appreciable antimicrobial activity against Gram negative bacteria but its antibacterial efficacy against Mycobacterium leprae has been reported.
Drug combination: Because microbes can easily develop resistance against the antimicrobial activity of fusidic acid, it is often recommended to use fusidic acid in combination with other similar antibacterial agents in order to avoid the development of resistance. And if fusidic acid should be used alone (i.e., as a monotherapy), higher doses of fusidic acid are recommended for better activity.
Fusidic acid can be used in combination with aminoglycosides (e.g., gentamicin and kanamycin). Fusidic acid produces a synergistic effect or additive effect when used in combination with rifampicin. But it produces antagonistic effect when used with quinolones; and thus it should not be used with these agents.
Contraindications for fusidic acid use: Fusidic acid has the ability to cross the placenta barrier in pregnant mothers. Therefore, fusidic acid is suspected to have possible teratogenic effects (i.e., birth defects) on the unborn foetus. It should be used with caution in pregnant mothers and in lactating or breastfeeding mothers. Always consult your physician before opting for the use of any antibiotic especially if you are pregnant or a nursing mother.
Side effects: The use of fusidic acid can cause mild liver problems such as jaundice (yellowing of the skin and the whites of the eyes) in its users. However, these symptoms disappear after completion of the therapy.
Pharmacokinetics of fusidic acid
In terms of its assimilation in the body, the absorption of Sodium fusidate is not very rapid. And skin penetration of fusidic acid is not too rapid, but slow. In terms of its distribution in the body, fusidic acid also has minimal or modest but efficient distribution in the body; and fusidic acid can achieve sufficient level of bioavailability in targeted pus infected regions of the body. In terms of its elimination from the body, fusidic acid is usually eliminated from the body via the liver, through the hepatic metabolism pathway.
References
Pfaller, M; Castaneira, M; Sader, H; Jones, R (2010). Evaluation of the activity of fusidic acid tested against contemporary Gram-positive clinical isolates from the USA and Canada. International Journal of Antimicrobial Agents. 35: 282–287.
Spelman. (1999). Fusidic acid in skin and soft tissue infections. International Journal of Antimicrobial Agents. 12 Suppl 2: S59–66.
A.J. O’Neill, F. McLaws, G. Kahlmeter, A. S. Henriksen, and I. Chopra (2007). Genetic Basis of Resistance to Fusidic Acid in Staphylococci. Antimicrobial Agents and Chemotherapy, 1737-1740.
Bode KA, Donner MG, Leier I, Keppler D (2002). Inhibition of transport across the hepatocyte canalicular membrane by the antibiotic fusidate. Biochem Pharmacol, 64:151-158.
Perry MJ, Hendricks-Gittins A, Stacey LM, Adlard MW, Noble WC (1983). Fusidane antibiotics produced by dermatophytes. J Antibiot, 36:1659-1663.
Reeves DS (1987). The pharmacokinetics of fusidic acid. J Antimicrob Chemother, 20:467-476.
Discover more from Microbiology Class
Subscribe to get the latest posts sent to your email.