Saccharomyces cerevisiae, commonly known as baker’s yeast or budding yeast, is a unicellular eukaryotic fungus that has been an integral part of human civilization for thousands of years. As a member of the Kingdom Fungi, genus Saccharomyces, and family Saccharomycetaceae, S. cerevisiae is renowned not only for its essential role in traditional food and beverage industries but also for its significance as a model organism in modern biological research.
Historically, S. cerevisiae has been utilized extensively in the production of bread, beer, and wine, where it facilitates fermentation by metabolizing sugars into carbon dioxide and alcohol. This process results in dough rising in baking and alcohol production during brewing and winemaking. The ability of S. cerevisiae to rapidly ferment carbohydrates under both aerobic and anaerobic conditions underscores its remarkable metabolic flexibility.
Morphologically, the yeast cells are typically round to ovoid, measuring between 5 and 10 micrometers in diameter. They propagate primarily through asexual budding, where a daughter cell forms as a small protrusion on the mother cell and eventually detaches. This efficient reproductive strategy contributes to its rapid growth and adaptability in diverse environments.
Nutritionally, S. cerevisiae thrives on simple carbohydrate sources such as glucose, maltose, and trehalose, utilizing these as sole carbon and energy sources. It also requires nitrogen sources like urea, amino acids, peptides, and ammonia, along with essential mineral elements including sulfur, phosphorus, iron, calcium, and magnesium for optimal growth and metabolism.
Beyond its traditional uses, S. cerevisiae has become a cornerstone in biotechnology and molecular biology. Its ease of cultivation, well-characterized genetics, and amenability to genetic manipulation have established it as one of the most intensively studied eukaryotic model organisms. It is widely employed in the production of biofuels, pharmaceuticals, and industrial enzymes, as well as in research exploring cellular processes such as gene regulation, protein folding, and metabolic pathways.
Saccharomyces cerevisiae remains an indispensable organism bridging ancient fermentation practices and cutting-edge biotechnological innovation, illustrating the profound interplay between tradition and science.
1. Introduction
Saccharomyces cerevisiae is a unicellular eukaryotic microorganism belonging to the kingdom Fungi. Taxonomically, it is classified within the phylum Ascomycota, class Saccharomycetes, order Saccharomycetales, family Saccharomycetaceae, and genus Saccharomyces. This yeast species is one of the most extensively studied fungi and holds a central place in both scientific research and various industrial applications. The genus name Saccharomyces originates from the Greek words “sakcharon” meaning sugar and “myces” meaning fungus, a nod to its remarkable ability to metabolize sugars by fermenting them into ethanol and carbon dioxide.
Historically, S. cerevisiae has been indispensable to human civilization for millennia, playing a crucial role in the production of bread, beer, and wine. Through these traditional uses, it has garnered several common names such as baker’s yeast, brewer’s yeast, top-fermenting yeast, and ale yeast, depending on its specific role and fermentation conditions. Its ability to efficiently convert fermentable sugars into carbon dioxide gas is critical for dough leavening in baking, and its alcohol-producing fermentation is fundamental to brewing and winemaking industries.
Beyond its industrial value, S. cerevisiae is highly regarded in molecular and cell biology as a premier model organism. Its eukaryotic cellular structure, genetic tractability, and rapid growth rate make it an ideal system for studying fundamental biological processes such as cell cycle regulation, metabolism, genetics, and protein synthesis. Consequently, S. cerevisiae bridges ancient biotechnology with modern molecular biology, representing a key organism in both applied and basic sciences.
2. Morphology and Growth Characteristics
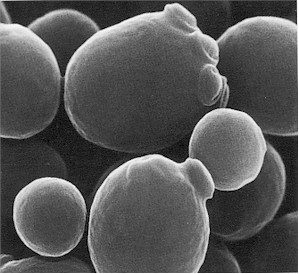
Saccharomyces cerevisiae cells exhibit distinct morphological features typical of budding yeasts. The cells are generally ellipsoidal to cylindrical in shape, often described as ovoid or spherical, with an average diameter ranging between 5 and 10 micrometers. Under the microscope, they appear as budding yeast cells with a groundnut shape (Figure 1). This size range facilitates microscopic observation and experimental manipulation. Each yeast cell is enveloped by a robust cell wall primarily composed of polysaccharides such as β-glucans and mannoproteins, with chitin concentrated at the bud scars and septa. This multilayered cell wall not only provides structural integrity and protection against environmental stresses but also plays a critical role in cellular interactions, adhesion, and biofilm formation.
Under optimal laboratory conditions, S. cerevisiae can be readily propagated on simple, nutrient-rich mycological media, including Sabouraud Dextrose Agar (SDA) and Potato Dextrose Agar (PDA). On these media, it typically forms creamy, smooth, and slightly pasty colonies with a characteristic white to off-white coloration. The yeast’s adaptability to various substrates allows it to thrive in diverse environments, ranging from natural fermentations to industrial bioreactors.
A hallmark feature of S. cerevisiae biology is its mode of asexual reproduction through budding. In this process, a small bud emerges asymmetrically from the mother cell, enlarging progressively until it detaches as a fully formed daughter cell. Bud scars, composed primarily of chitin, remain on the mother cell surface and serve as molecular markers to determine the replicative age of the cell—a key parameter in aging and cellular senescence studies. This unique reproductive mechanism enables rapid population expansion, contributing to its widespread success both in nature and industrial applications.
3. Nutritional Requirements and Metabolism
Saccharomyces cerevisiae is a facultative anaerobe, capable of growing both in the presence and absence of oxygen. It thrives on simple carbohydrates like glucose, maltose, and trehalose, which serve as its main carbon and energy sources. Notably, S. cerevisiae does not metabolize lactose or cellobiose due to the absence of requisite enzymes.
For nitrogen, the yeast utilizes a variety of sources including ammonium salts, amino acids, peptides, and urea. Additionally, the yeast requires essential minerals such as phosphorus, sulfur, potassium, magnesium, iron, and calcium for optimal metabolic function and cellular development. Vitamins such as biotin, pantothenic acid, and inositol also play significant roles in yeast physiology.
The metabolic versatility of S. cerevisiae is exemplified by its ability to undergo alcoholic fermentation in anaerobic conditions and oxidative respiration in the presence of oxygen. Under fermentative conditions, sugars are converted primarily to ethanol and carbon dioxide, a trait that underpins its use in beverage and bread production.
4. Genetic and Molecular Attributes
Saccharomyces cerevisiae was the first eukaryotic organism to have its entire genome sequenced, consisting of approximately 12 million base pairs and around 6,000 genes distributed across 16 chromosomes. This genomic information has greatly facilitated studies in genetics, molecular biology, and systems biology.
Due to its genetic tractability, S. cerevisiae is amenable to transformation techniques allowing for insertion, deletion, and expression of foreign genes. This has made it a cornerstone organism for synthetic biology, gene expression studies, and metabolic engineering. Scientists can manipulate its genome using CRISPR-Cas systems, homologous recombination, and other genetic tools to produce valuable metabolites, enzymes, and recombinant proteins.
5. Industrial Applications
The industrial significance of Saccharomyces cerevisiae is immense and multifaceted, spanning numerous biotechnological, food, and pharmaceutical sectors. As one of the most widely utilized microorganisms, it plays a pivotal role in traditional fermentative processes such as bread making, beer brewing, and wine production. In bread making, S. cerevisiae acts as a natural leavening agent by fermenting sugars to produce carbon dioxide, which causes the dough to rise, yielding a soft, airy texture in the final product. In the brewing industry, different strains of S. cerevisiae are used to produce top-fermented ales, where the yeast ferments at warmer temperatures and contributes distinct flavors and aromas.
Beyond food and beverage industries, S. cerevisiae is instrumental in bioethanol production, serving as a bio-catalyst that ferments sugars derived from biomass into ethanol fuel. Additionally, it is used in the production of industrial alcohols, spirits, and feed yeasts, which are valuable protein-rich supplements in animal nutrition. Advances in genetic engineering have further expanded its industrial applications, enabling the yeast to produce pharmaceuticals, enzymes, and bio-based chemicals. Its ability to adapt to diverse substrates, coupled with a high yield capacity, underscores S. cerevisiae’s critical role as an industrial workhorse in biotechnology.
Table 1. Overview of some applications of Saccharomyces
| Yeast Strain | Product/Application |
| Saccharomyces cerevisiae | Bread |
| S. cerevisiae | Ethanol |
| S. bayanus | Sparkling wine |
| S. cerevisiae | Top-fermenting beer |
| S. cerevisiae var. ellipsoideus | Wine |
| S. uvarum | Bottom-fermenting beer |
| S. cerevisiae | Spirits |
| S. cerevisiae | Feed and food yeasts |
5.1 Baking Industry
In the baking industry, Saccharomyces cerevisiae plays a pivotal role as a natural leavening agent, essential for the production of bread and other yeast-leavened baked goods. The fundamental biological process underlying this role is fermentation, wherein S. cerevisiae metabolizes fermentable sugars found in the flour dough, such as glucose and maltose, converting them into carbon dioxide (CO₂) and ethanol. This metabolic activity is primarily anaerobic but can also occur in aerobic conditions when sugar is abundant.
The carbon dioxide gas produced during fermentation accumulates within the dough matrix, creating microscopic bubbles that expand and cause the dough to rise. This leavening effect gives bread its characteristic light, airy, and porous crumb structure. Without this yeast-driven fermentation, bread dough would remain dense and compact, lacking the desirable texture consumers expect. Meanwhile, the ethanol generated as a byproduct of fermentation volatilizes during the baking process, contributing subtle flavor notes but largely evaporates due to the high temperatures involved. The heat from baking ultimately kills the yeast cells, ensuring they do not remain active in the finished product.
While many people associate bread rising with chemical leavening agents such as sodium bicarbonate (commonly called baking soda), it is important to distinguish that in traditional yeast-leavened breads, S. cerevisiae is the biological catalyst responsible for the fermentation process. Chemical leavening agents rely on acid-base reactions to produce CO₂ quickly, which is typical in products like cakes and biscuits. In contrast, yeast fermentation is a slower, controlled process that not only affects texture but also develops complex flavors and aromas unique to yeast-leavened bread.
Moreover, S. cerevisiae has been selectively bred and optimized over centuries for baking purposes, resulting in strains known as “baker’s yeast.” These strains are characterized by rapid fermentation rates, high CO₂ output, and tolerance to various environmental stresses such as osmotic pressure and temperature fluctuations. Baker’s yeast is commercially available as active dry yeast, instant yeast, or fresh compressed yeast, providing bakers with flexible options to suit different production scales and processes.
The versatility of S. cerevisiae in baking extends beyond bread to include products such as pizza dough, rolls, croissants, and other fermented dough goods. Its ability to metabolize sugars rapidly and efficiently, coupled with its contribution to flavor and texture, cements its status as a cornerstone organism in the baking industry.
5.2 Alcoholic Beverages
Saccharomyces cerevisiae is equally indispensable in the production of a wide variety of alcoholic beverages, including beer, wine, and distilled spirits. Its role is to convert sugars present in raw materials—such as malted barley in beer and grape must in wine—into ethanol and a complex mixture of secondary metabolites that influence aroma, flavor, and mouthfeel.
In beer brewing, S. cerevisiae is primarily employed as a top-fermenting yeast. This means the yeast cells tend to rise to the surface of the fermentation vessel during the process, operating optimally at relatively warmer temperatures between 15–24°C. This fermentation style is characteristic of ale production, which includes popular beer styles such as pale ales, stouts, and IPAs (India Pale Ales). The warmer fermentation and metabolic pathways of S. cerevisiae result in the formation of fruity esters and phenolic compounds, which contribute to the distinctive and complex flavor profiles associated with ales.
Conversely, lagers are brewed using bottom-fermenting yeasts such as Saccharomyces uvarum (formerly classified as S. carlsbergensis), which function at cooler temperatures (8–12°C) and settle at the bottom of the fermentation vessel. The slower fermentation process and lower temperature reduce ester production, resulting in cleaner, crisper beer flavors typical of lagers.
In winemaking, S. cerevisiae and its close relative Saccharomyces bayanus are the predominant yeasts responsible for the alcoholic fermentation of grape must. The natural sugars in grapes—primarily glucose and fructose—are metabolized by the yeast to produce ethanol and carbon dioxide, alongside a suite of volatile compounds including higher alcohols, esters, acids, and aldehydes. These metabolites profoundly influence the sensory attributes of wine, affecting its bouquet, taste, and mouthfeel.
Winemakers carefully select yeast strains based on their fermentation kinetics, tolerance to alcohol and sulfites, and flavor production profiles. Some strains are prized for producing floral and fruity esters, while others contribute more neutral or balanced flavors. Additionally, S. cerevisiae’s robust fermentation capacity ensures complete sugar utilization, critical for achieving desired alcohol levels and preventing spoilage by undesirable microbes.
In the production of distilled spirits such as whiskey, vodka, and brandy, S. cerevisiae initiates fermentation of the base mash or must, converting sugars into ethanol. The ethanol is then concentrated through distillation to produce the final high-proof spirit. The yeast’s fermentation behavior influences the flavor precursors available for maturation and aging processes, ultimately impacting the character of the distilled product.
Overall, Saccharomyces cerevisiae’s adaptability, fermentation efficiency, and capacity to generate diverse flavor compounds have made it a fundamental organism in the beverage alcohol industry worldwide, a tradition that dates back thousands of years.
5.3 Bioethanol and Biofuel Production
Saccharomyces cerevisiae has emerged as a cornerstone microorganism in the production of bioethanol, a renewable biofuel that presents a promising sustainable alternative to conventional fossil fuels. Bioethanol is primarily produced via the fermentation of sugars, and S. cerevisiae’s natural fermentative capacity makes it an ideal organism for this purpose. Traditionally, first-generation bioethanol is derived from sugar- and starch-rich crops such as sugarcane, corn, and wheat. However, concerns about food security and land use have propelled the development of second-generation bioethanol technologies that utilize lignocellulosic biomass — agricultural residues, woody materials, and grasses — as feedstock.
Lignocellulosic biomass is a complex matrix of cellulose, hemicellulose, and lignin. While cellulose and hemicellulose can be hydrolyzed into fermentable sugars, many conventional yeast strains cannot efficiently metabolize pentose sugars such as xylose and arabinose that are abundant in hemicellulose. To overcome this limitation, bioengineers have developed genetically modified strains of S. cerevisiae capable of fermenting both hexose (e.g., glucose) and pentose sugars, significantly improving the efficiency and economic viability of bioethanol production from lignocellulosic feedstocks.
These engineered strains employ heterologous expression of genes encoding pentose transporters and metabolic enzymes, enabling the uptake and conversion of pentoses into ethanol. Furthermore, adaptive laboratory evolution and CRISPR-Cas9 genome editing techniques have accelerated the optimization of yeast strains for tolerance to fermentation inhibitors such as furfural and acetic acid, which are generated during biomass pretreatment.
The ability of S. cerevisiae to ferment a broad spectrum of sugars under anaerobic conditions, coupled with its robustness in industrial-scale fermentations, makes it indispensable in advancing biofuel technologies. Beyond ethanol, ongoing research is exploring yeast strains engineered to produce other biofuels and biochemicals, including butanol, isobutanol, and advanced bio-hydrocarbons, positioning S. cerevisiae at the forefront of sustainable energy solutions.
5.4 Biotechnology and Pharmaceuticals
In the realm of biotechnology and pharmaceutical manufacturing, Saccharomyces cerevisiae plays a pivotal role as a eukaryotic expression system for the production of recombinant proteins, vaccines, and industrially relevant enzymes. One of the most notable success stories is the production of the hepatitis B vaccine, which relies on genetically engineered S. cerevisiae cells to express the hepatitis B surface antigen (HBsAg). This recombinant protein undergoes proper folding and essential post-translational modifications in yeast, ensuring its immunogenicity and safety for human use.
Unlike prokaryotic systems such as Escherichia coli, S. cerevisiae possesses the eukaryotic cellular machinery required for complex post-translational modifications including glycosylation, disulfide bond formation, and proteolytic processing, which are critical for the biological activity of many therapeutic proteins. This advantage makes yeast a preferred host for producing biopharmaceuticals such as insulin, interferons, and monoclonal antibodies in research and clinical settings.
Moreover, S. cerevisiae has demonstrated considerable utility in bioremediation applications. Its metabolic versatility and tolerance to toxic compounds allow it to degrade environmental pollutants including heavy metals and xenobiotics, thereby contributing to sustainable waste management strategies.
The yeast’s capacity for synthesizing bioplastics such as polyhydroxyalkanoates (PHAs) is also gaining attention as a promising route to replace petroleum-based plastics. Genetic engineering efforts have tailored S. cerevisiae to produce these biodegradable polymers efficiently, underscoring its growing importance in the development of circular bioeconomies.
Together, these applications highlight S. cerevisiae as a multifaceted microorganism at the intersection of industrial biotechnology and environmental sustainability, continuously driving innovations that address pressing global challenges in health, energy, and ecology.
6. Advantages of Using Saccharomyces cerevisiae in Research and Industry
Saccharomyces cerevisiae has long been recognized as one of the most valuable organisms in both scientific research and industrial applications. Its widespread use is driven by a combination of biological, practical, and regulatory advantages that make it uniquely suited for a broad range of purposes.
Rapid Growth and High Biomass Production: One of the foremost advantages of S. cerevisiae is its remarkably short doubling time, often ranging between 90 to 120 minutes under optimal conditions. This rapid growth allows researchers and industrial producers to obtain large quantities of yeast biomass in a relatively short period. This efficiency not only accelerates laboratory experiments but also enables cost-effective large-scale fermentations.
Simple Nutritional Requirements: Unlike many other eukaryotic cells, S. cerevisiae has relatively minimal nutritional demands. It can grow robustly on inexpensive, defined media containing basic carbon sources such as glucose or maltose, and nitrogen sources like ammonia or amino acids. This simplicity reduces the cost of cultivation and facilitates scalability in industrial processes.
Ease of Genetic Manipulation: The yeast has a well-characterized genome and a comprehensive suite of genetic tools, including plasmid vectors, gene knockout and knock-in systems, and CRISPR-Cas9 based editing. This genetic tractability enables researchers to easily modify yeast strains for experimental purposes or to optimize production strains for specific industrial outputs, such as bioethanol, pharmaceuticals, or recombinant proteins.
A Model Eukaryotic Organism: As a unicellular eukaryote, S. cerevisiae serves as a powerful model for studying fundamental cellular processes common to higher organisms, including humans. Research in yeast has contributed to understanding DNA replication, cell cycle control, metabolism, and aging, making it invaluable for molecular and cellular biology.
Generally Recognized As Safe (GRAS): Regulatory authorities worldwide, including the U.S. Food and Drug Administration (FDA), classify S. cerevisiae as GRAS. This status is crucial for its extensive use in food production—such as baking and brewing—and pharmaceutical manufacturing, where safety is paramount.
High Stress Tolerance: Industrial fermentations often involve harsh conditions such as high ethanol concentrations, osmotic pressure, and temperature fluctuations. Certain S. cerevisiae strains have evolved or been engineered to tolerate these stresses, maintaining metabolic activity and productivity under adverse environments. This resilience enhances process stability and product yields.
Together, these attributes make Saccharomyces cerevisiae a remarkably versatile and indispensable organism in both fundamental research and numerous biotechnological industries, underpinning innovations in health, nutrition, and sustainable manufacturing.
7. Limitations and Future Directions
Despite its many advantages, S. cerevisiae has limitations. It lacks the ability to naturally utilize certain substrates like lactose or cellulose. However, metabolic engineering and synthetic biology have enabled the modification of S. cerevisiae strains to broaden their substrate range.
Future directions include:
- Engineering strains for higher ethanol tolerance and productivity.
- Expanding its use in pharmaceutical manufacturing (e.g., for monoclonal antibodies).
- Using yeast artificial chromosomes (YACs) for cloning large DNA segments.
- Integrating systems biology approaches to optimize metabolic pathways.
- Exploring its role in probiotics and gut microbiome research.
8. Conclusion
Saccharomyces cerevisiae is more than a microorganism used in baking and brewing; it is a cornerstone of modern biotechnology. Its unique combination of eukaryotic features, ease of cultivation, and genetic manipulability make it a powerful tool in science and industry. From ancient fermentation processes to cutting-edge gene therapy, S. cerevisiae has proven to be an indispensable ally in humanity’s technological advancement. With continued research and innovation, its applications will likely expand into even more domains, including sustainable energy, biopharmaceuticals, and beyond.
References
Bader F.G (1992). Evolution in fermentation facility design from antibiotics to recombinant proteins in Harnessing Biotechnology for the 21st century (eds. Ladisch, M.R. and Bose, A.) American Chemical Society, Washington DC. Pp. 228–231.
Nduka Okafor (2007). Modern industrial microbiology and biotechnology. First edition. Science Publishers, New Hampshire, USA.
Das H.K (2008). Textbook of Biotechnology. Third edition. Wiley-India ltd., New Delhi, India.
Latha C.D.S and Rao D.B (2007). Microbial Biotechnology. First edition. Discovery Publishing House (DPH), Darya Ganj, New Delhi, India.
Nester E.W, Anderson D.G, Roberts C.E and Nester M.T (2009). Microbiology: A Human Perspective. Sixth edition. McGraw-Hill Companies, Inc, New York, USA.
Steele D.B and Stowers M.D (1991). Techniques for the Selection of Industrially Important Microorganisms. Annual Review of Microbiology, 45:89-106.
Pelczar M.J Jr, Chan E.C.S, Krieg N.R (1993). Microbiology: Concepts and Applications. McGraw-Hill, USA.
Prescott L.M., Harley J.P and Klein D.A (2005). Microbiology. 6th ed. McGraw Hill Publishers, USA.
Steele D.B and Stowers M.D (1991). Techniques for the Selection of Industrially Important Microorganisms. Annual Review of Microbiology, 45:89-106.
Summers W.C (2000). History of microbiology. In Encyclopedia of microbiology, vol. 2, J. Lederberg, editor, 677–97. San Diego: Academic Press.
Talaro, Kathleen P (2005). Foundations in Microbiology. 5th edition. McGraw-Hill Companies Inc., New York, USA.
Thakur I.S (2010). Industrial Biotechnology: Problems and Remedies. First edition. I.K. International Pvt. Ltd. New Delhi, India.
Discover more from Microbiology Class
Subscribe to get the latest posts sent to your email.