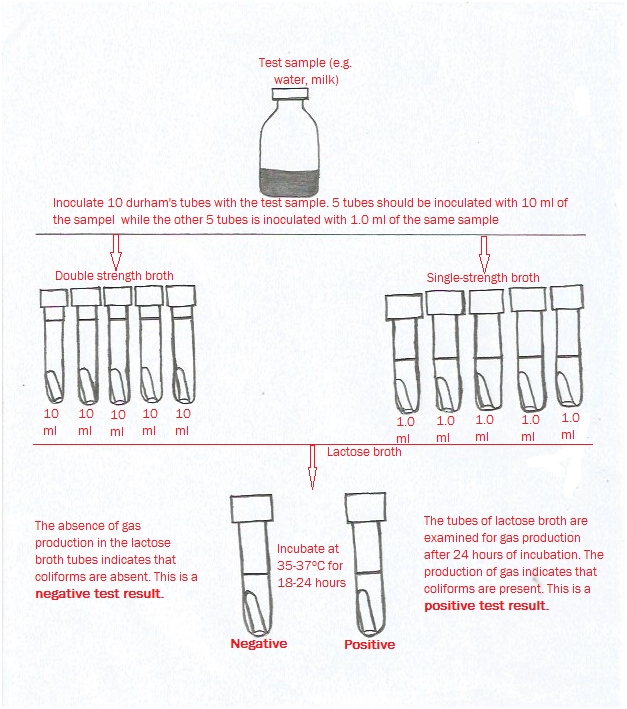
Water quality is defined as the suitability of water to sustain various uses or processes without causing any untoward effect to its users. Several human and natural factors influences the quality of water available for many industrial and domestic applications; and thus it is vital to continuously monitor water for various uses and processes – in order to ensure their conformity to certain criteria that certify their quality and safety for use.
According to the International Organization for Standardization (ISO), water monitoring is defined as the programmed process of sampling, measurement and subsequent recording or signaling, or both, of various water characteristics, often with the aim of assessing conformity to specified objectives. The mina objective of water quality management is to control the physical, chemical, and biological features or factors of water. Aside air, other sources of microbial contamination in a manufacturing industry may include personnel, equipment, manufacturing facility, vermin’s (e.g. rodents and insects) and water.
Water is a critical resource for every manufacturing process. And since safety and water quality is a serious issue in today’s manufacturing industries including food industries, medical industries and pharmaceutical industries, it is important to monitor and ascertain the level of microbial contamination of the water used for manufacturing processes. The presence of infectious disease agents including bacteria, fungi and viruses in water used for manufacturing pose a severe safety and health risk to the finished products and the consumers or users of the products from manufacturing industries.
Colossal economic loss to these industries resulting from product recall and litigations could lead to loss of profit and decrease in revenue generation not to talk of the loss of the company’s image in the global or local market. Aside this, the contaminated product may cause infection or disease in the population of people using the product; and this will lead to loss of quality time for work and possible hospitalization of the sick patients. Thus, proper monitoring of water quality is vital to assuage any economic loss that may result from the use of contaminated water for production. Water is used for various processes during manufacturing including washing of raw materials and equipment, and water is also used for the preparation of the constituents used for compounding the finished products.
The water used for the manufacturing of pharmaceutical preparations must be found to be of good quality and free from feacal, chemical or microbial contamination. Water meant for manufacturing should be properly sterilized or treated to reduce its microbial load to the minimal level deemed fit for the manufacturing process. Water for industrial production may be sourced from surface waters and groundwater; and these waters are evaluated for the most important physical, chemical and biological and/or microbiological parameters that may affect the quality of the water.
Surface water bodies may be contaminated directly with heavy metals such as lead from smelting and mining activities. Acidification of surface waters due to the combination of gases from burning of fossil fuels and moisture in the atmosphere can also cause the contamination of surface waters – when the moisture in the atmosphere falls as acid rain. Leaching from landfills and discharge of industrial effluents (especially without proper treatment) can also contribute to the contamination of surface waters. Groundwater systems can also be contaminated by leachate from mining sites, solid waste dump sites and from other heavy industrial production sites.
Water is vitally important to every aspect of our lives and it is used for domestic activities and for other industrial or manufacturing processes. Thus, monitoring the quality of surface water will help protect our waterways from pollution. Water quality can be difficult to measure – since water is a vast network of branching rivers, springs, swamps, estuaries, wetlands, and lakes. And this makes each water body to contain different levels of pollution and different physical, chemical and biological properties. Water quality issues influence human and environmental health, so the more we monitor our water the better we will be able to recognize and prevent contamination problems.
Water that is to be used for certain industrial production especially those that involve the production of sterile parenteral, vaccines and drugs should be free from chemicals or microorganisms that could pose health risk to humans or animals. Microorganisms are ubiquitous and can be found everywhere including water. Aside microbes, water can also contain heavy metals and other toxic chemical substances that may adversely affect human health.
Some of the physicochemical parameters that determine water quality include turbidity, odour, taste, colour, pH, conductivity, salt concentration, alkalinity, acidity and the concentration of suspended solids. Water for industrial production is monitored for any of these parameters including the microbial community present in the water body – in order to ensure that the water used for manufacturing is safe and pose no health risk to the general population.
References
Arora D.R (2004). Quality assurance in microbiology. Indian J Med Microbiol, 22:81-86.
Ashutosh Kar (2008). Pharmaceutical Microbiology, 1st edition. New Age International Publishers: New Delhi, India.
Axelsen P.H (2002). Essentials of antimicrobial pharmacology. Humana Press, Totowa, New Jersey, USA. Al-Jasser A.M (2006). Extended – Spectrum Beta – Lactamases (ESBLs): A Global Problem. Kuwait Medical Journal, 38(3):171-185.
Bisht R., Katiyar A., Singh R and Mittal P (2009). Antibiotic Resistance – A Global Issue of Concern. Asian Journal of Pharmaceutical and Clinical Research, 2 (2):34-39.
Block S.S (2001). Disinfection, sterilization and preservation. 5th edition. Lippincott Williams & Wilkins, Philadelphia and London.
Joslyn, L. J. (2000). Sterilization by Heat. In S. S. Block (Ed.), Disinfection, Sterilization, and Preservation (5th ed., pp. 695-728). Philadelphia, USA: Lippincott Williams and Wilkins.
Nally J.D (Ed.) (2007). Good manufacturing practices for pharmaceuticals. Sixth edition. Informa Healthcare USA, Inc, New York.
Discover more from Microbiology Class
Subscribe to get the latest posts sent to your email.