Metabolism is simply defined as the summation of the chemical reactions that occurs in the cell at each point in time. It is the processes of catabolism (i.e. breaking down of molecules) and anabolism (i.e. the building up of newer molecules) that occur in the cell. Metabolism is critical for the management of an organism’s energy sources and other cellular materials or products. The term metabolism was originally invented by the famous German physiologist, Theodor Schwann (1810-1882) to mean all of an organism’s chemical processes. It is noteworthy that all living cells depend on a series of biochemical reactions that go on in the cell in order to maintain homeostasis (i.e. a constant internal environment).
Metabolic reactions in the form of oxidation and reduction reactions always occur in microbial cells; and these activities direct the synthesis of important molecules for the cells growth, reproduction and development via various metabolic pathways catalyzed by enzymatic reactions. Oxidation-reduction reaction (or redox reaction) is a type of reaction that occur in living systems in which electrons are transferred from one substance or molecule to another especially in scenarios where energy is either released for cell activities or for storage purposes.
In redox reactions, electrons flow from reducing agents (the electron donors or reductants) to oxidizing agents (the electron acceptors or oxidants); and the entire oxidation reaction (i.e. redox reaction) is reversible in nature. Free energy in the form of ATP is always released for cellular activities each time electrons flow from reductants to oxidants during redox reactions in the cell.Metabolic reactions in the cell ensure that complex organic molecules (e.g. carbohydrates and lipids) are broken-down to simpler molecules (e.g. CO2 and NH4+) that can be utilized by the cell for its normal activities.
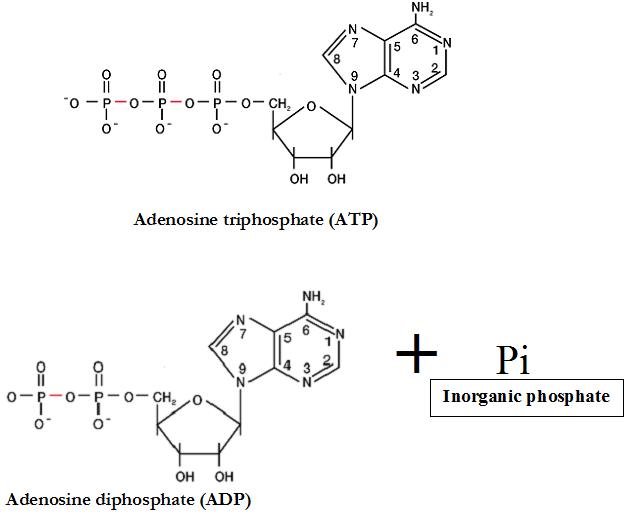
In majority of these reactions, molecules known as electron acceptors are reduced while the electron donors become oxidized. Generally, chemical reactions in the cell i.e. redox reactions can either require energy or release energy; and these processes are generally known as oxidation-reduction reactions as earlier highlighted. And the energy currency of the cell is known as adenosine triphosphate (ATP), a nucleotide molecule that has three phosphate groups linked to a pentose sugar by phosphodiester bonds. On hydrolysis, ATP (the principal energy-rich chemical of the cell) is converted to adenosine diphosphate (ADP) and inorganic phosphate (Pi), and this process marks the release of energy in the cell (Figure 1).
The energy released during the oxidation of carbon molecules and other complex organic molecules is captured and utilized for the synthesis of ATP from ADP and inorganic phosphate molecules. And the energy required for chemical reactions in the cell as well as those released during redox reaction is stored in the form of ATP. And once energy is needed, ATP is hydrolyzed and free energy is released for metabolic activities in the cell. ATP is the major link between catabolism and anabolism. Just as money is earned and spent in an economy, ATP (which is the energy currency of the cell) is also produced or earned in catabolic reactions and expended or consumed (i.e. utilized) in anabolic reactions for the overall growth and development of the cell.
During catabolic reactions, complex molecules such as proteins, starch or carbohydrates and lipids are broken down to simpler molecules including amino acids, glucose and glycerol or fatty acids respectively. The energy required for this hydrolytic reaction is from ATP; and anabolic reactions produce energy which is transferred to catabolic pathways for the breakdown of complex molecules in the cell. On the other hand, the energy released during catabolic reactions is stored in the cell as ATP (the energy currency of the cell).
References
Alberts B, Bray D, Lewis J, Raff M, Roberts K and Watson J.D (2002). The molecular Biology of the Cell. Fourth edition. New York, Garland, USA.
Berg JM, Tymoczko JL, Stryer L (2002). Biochemistry (5th ed.). New York, NY: W. H. Freeman.
Brooks G.F., Butel J.S and Morse S.A (2004). Medical Microbiology, 23rd edition. McGraw Hill Publishers. USA.
Campbell, Neil A.; Brad Williamson; Robin J. Heyden (2006). Biology: Exploring Life. Boston, Massachusetts: Pearson Prentice Hall.
Cooper G.M and Hausman R.E (2004). The cell: A Molecular Approach. Third edition. ASM Press.
Dale J (2003). Molecular genetics of bacteria. Jeremy W. Dale and Simon Park (4th eds.). John Wiley & Sons Ltd, West Sussex, UK. Pp.
Karp, Gerald (2009). Cell and Molecular Biology: Concepts and Experiments. John Wiley & Sons.
Lodish H, Berk A, Matsudaira P, Kaiser C.A, Kreiger M, Scott M.P, Zipursky S.L and Darnell J (2004). Molecular Cell Biology. Fifth edition. Scientific American Books, Freeman, New York, USA.
Madigan M.T., Martinko J.M., Dunlap P.V and Clark D.P (2009). Brock Biology of microorganisms. 12th edition. Pearson Benjamin Cummings Publishers. USA. Pp.795-796.
Maton, Anthea (1997). Cells Building Blocks of Life. New Jersey: Prentice Hall.
Discover more from Microbiology Class
Subscribe to get the latest posts sent to your email.