Antibiotics remain one of the most important discoveries in medical science, serving as a cornerstone in the fight against infectious diseases from time immemorial. Among them, Streptomycin occupies a special place in history. Streptomycin is an aminoglycoside antibiotic originally isolated from the actinobacterium Streptomyces griseus, a soil-dwelling bacterium. Streptomyces species thrive in rich organic soils, where they secrete streptomycin as a secondary metabolite to inhibit competing bacteria. Through laboratory isolation and fermentation, the antibiotic can be extracted from the cultured soil microorganisms and purified for medical use. The discovery of streptomycin in 1943 marked the first effective treatment for tuberculosis, a disease that had caused significant morbidity and mortality worldwide. Beyond its role in tuberculosis therapy, streptomycin has proven to be effective against a wide range of Gram-negative bacterial pathogens, including Escherichia coli, Klebsiella pneumoniae, and Pseudomonas aeruginosa. It functions by binding to the 30S ribosomal subunit of bacteria, interfering with protein synthesis and leading to cell death.
The genus Streptomyces is particularly notable for its remarkable ability to produce diverse secondary metabolites with antimicrobial, antifungal, and antitumor activities. It is estimated that more than two-thirds of naturally derived antibiotics originate from Streptomyces species. Secondary metabolites, unlike primary metabolites essential for growth and reproduction, are typically synthesized under specific environmental conditions or during stationary growth phases of microbial cells. These metabolites confer a competitive advantage in the microbial ecosystem by inhibiting rival microorganisms. Streptomycin exemplifies such a metabolite, and its extraction and evaluation provide an excellent educational model for understanding natural product discovery, microbial ecology, and applied microbiology.
The extraction of antibiotics like streptomycin from microbial cultures involves cultivating the producing organism under suitable conditions, followed by recovery and partial purification of the bioactive compound from the fermentation broth. Evaluation of antimicrobial activity is then commonly conducted using in vitro assays such as the agar well diffusion method. In this method, test organisms are inoculated onto an agar plate, and wells are filled with the extracted antibiotic. Following incubation, zones of inhibition surrounding the wells indicate the antibiotic’s potency and spectrum of activity of the isolated bioactive compound.
Extraction of streptomycin
The production of streptomycin begins with the cultivation of Streptomyces griseus on nutrient-rich media, typically starch casein agar or other actinomycete-specific formulations. The organism is allowed to grow under aerobic conditions, as oxygen is critical for the biosynthesis of its secondary metabolites. Over time, the culture produces streptomycin, which diffuses into the surrounding medium.
To extract streptomycin, the fermentation broth is harvested after sufficient incubation. The culture is filtered to remove microbial cells, leaving a cell-free supernatant containing dissolved metabolites. Solvent extraction or precipitation methods are then employed to isolate the antibiotic. For instance, streptomycin can be precipitated by adjusting the pH and then collected via centrifugation or filtration technique. Additional purification steps, such as solvent partitioning or column chromatography, may be used in research laboratories to obtain a more refined product of the bioactive compound.
Microbial evaluation using the agar well diffusion method
Once extracted, the antimicrobial potential of streptomycin is evaluated against selected test organisms. The agar well diffusion method is one of the most widely used and straightforward assays for this purpose. In this technique, nutrient agar plates are seeded with a standardized inoculum of a bacterial test strain. Wells are then bored into the agar and filled with known volumes of the antibiotic extract.
The plates are incubated under optimal conditions for the growth of the test organism. During incubation, the antibiotic diffuses radially through the agar. If the test organism is susceptible to streptomycin, its growth will be inhibited in the vicinity of the well, resulting in a clear, circular zone of inhibition. The diameter of this zone can be measured and compared to reference values or controls to determine the relative effectiveness of the extracted bioactive compound.
Common test organisms used in such assays include both Gram-positive and Gram-negative bacteria, such as Staphylococcus aureus, E. coli, and Bacillus subtilis. Streptomycin is particularly active against Gram-negative bacteria, though some Gram-positive strains may also show susceptibility.
Significance of the experiment to extract streptomycin
The extraction and evaluation of streptomycin provide a practical illustration of several important microbiological and pharmacological principles.
- First, it highlights the natural role of soil microorganisms as prolific producers of antibiotics and underscores the importance of microbial ecology in drug discovery.
- Second, it demonstrates the experimental workflow from microbial cultivation, metabolite extraction, and antimicrobial screening – steps that are foundational in pharmaceutical microbiology.
- Third, the use of the agar well diffusion method introduces students and researchers to a simple yet powerful bioassay technique for measuring antimicrobial activity of (newly isolated) bioactive compounds.
This experiment also has broader implications in the context of modern challenges. With the rise of antimicrobial resistance (AMR), the discovery of new antibiotics or the reevaluation of existing ones is critical. Although streptomycin has been in use for decades and resistance to it is widespread, revisiting such natural products serves as a reminder of the microbial world’s potential as a reservoir of bioactive compounds. Moreover, the methodologies learned through this experiment can be applied to screen for novel antibiotics from environmental isolates, which is particularly important in an era where drug-resistant infections threaten global health.
Streptomycin, a pioneering antibiotic from Streptomyces griseus, exemplifies the importance of microbial secondary metabolites in medicine. Through its extraction and subsequent evaluation using the agar well diffusion method, one can appreciate not only its antimicrobial activity but also the broader processes involved in antibiotic discovery and testing. This experiment offers a valuable educational platform, reinforcing the link between microbiology, natural products chemistry, and clinical therapeutics, while also emphasizing the continuing relevance of antibiotic research in addressing contemporary health challenges.
Aim of the experiment
The aim of this experiment is to extract streptomycin from a culture of Streptomyces griseus and evaluate its antimicrobial activity against selected bacterial strains. This process demonstrates both the natural production of antibiotics by soil microorganisms and their effectiveness against clinically relevant pathogens.
Materials and methods required for streptomycin extraction
Materials
- Streptomyces griseus culture
The antibiotic-producing microorganism, S. griseus, is the source of streptomycin. Its ability to synthesize secondary metabolites makes it an ideal candidate for antibiotic extraction studies. S. griseus is isolated from soil in the following steps:
Isolation of Streptomyces griseus from Soil
- Sample Collection
- Collect soil samples (preferably from decayed organic matter–rich areas, e.g., compost, forest soil, or agricultural fields).
- Air-dry the soil sample at room temperature for 24 – 48 hours to reduce fast-growing bacterial and fungal populations.
- Serial Dilution
- Weigh 1 g of dried soil and suspend in 9 mL of sterile distilled water (10⁻¹ dilution).
- Perform serial dilutions up to 10-5 or 10-6 to reduce microbial load.
- Plating on Selective Media
- Plate 0.1 mL from appropriate dilutions onto selective media such as starch casein agar, glycerol-asparagine agar, or actinomycete isolation agar.
- To suppress contaminants, supplement media with antifungal agents (e.g., nystatin or cycloheximide) and antibacterial agents (e.g., nalidixic acid).
- Incubation
- Incubate plates at 28 – 30°C for 7 – 14 days.
- Observe for slow-growing, chalky, leathery, or powdery colonies typical of Streptomyces.
- Isolation of Colonies
- Pick well-isolated colonies with characteristic morphology.
- Re-streak on fresh selective agar plates to obtain pure cultures.
- Confirmation of isolate
- Examine colony morphology, pigmentation, and spore formation microscopically.
- Conduct biochemical and molecular identification (e.g., 16S rRNA sequencing) if required to confirm S. griseus.
- Maintenance of S. griseus culture
- Maintain pure cultures on starch casein agar slants at 4°C for short-term use.
- For long-term preservation, prepare glycerol stocks (20 – 30%) and store at -20°C or -80°C.
- Starch casein broth
This specialized medium provides starch as a carbon source and casein as a nitrogen source, supporting optimal growth of actinomycetes and stimulating the production of secondary metabolites like streptomycin.
- Rotary shaker
Used to maintain the culture in constant motion, the shaker ensures adequate aeration and mixing, both of which are essential for maximizing antibiotic yield.
- Whatman No. 1 filter paper
This filter paper is employed to separate microbial cells from the culture supernatant, allowing the antibiotic-containing liquid fraction to be collected.
- Ethyl acetate or n-butanol
These organic solvents facilitate the extraction of streptomycin from the aqueous broth by partitioning bioactive compounds into the solvent layer.
- Separating funnel
A separating funnel allows efficient separation of the aqueous and organic phases after solvent extraction, enabling recovery of the antibiotic-rich fraction.
- Rotary evaporator (or water bath)
The solvent is removed under reduced pressure or gentle heating, leaving behind concentrated crude streptomycin extract suitable for testing.
- Petri dishes
Sterile Petri dishes provide the platform for preparing agar plates, which will be used to grow test bacteria and assess antibiotic activity.
- Mueller-Hinton Agar (MHA)
This standardized medium is widely used in antimicrobial susceptibility testing because it supports reproducible growth of a variety of bacterial species.
- Test organisms: Pathogenic Escherichia coli, Staphylococcus aureus, Pseudomonas aeruginosa
These bacterial strains represent common human pathogens. They were chosen to demonstrate the activity spectrum of streptomycin against both Gram-negative and Gram-positive organisms.
- Sterile cork borer
A cork borer is used to create uniform wells in agar plates, ensuring consistency in the antimicrobial diffusion assay.
- Sterile pipettes and tips
Pipettes and tips are required for transferring precise volumes of antibiotic extract into the agar wells, preventing contamination and ensuring accuracy.
- Sterile distilled water
Serves as a diluent and solvent in the experiment, ensuring that all reagents and preparations remain contamination-free.
- Incubator (37°C)
The incubator provides an optimal temperature for bacterial growth, mimicking the human body environment where these pathogens typically thrive.
- Positive control: Standard streptomycin
Including a reference antibiotic ensures that the test results can be validated and compared to known activity levels.
- Negative control: Solvent only
The negative control ensures that any observed inhibition is due to the antibiotic itself, and not to the solvent used during extraction.
Steps in the extraction of streptomycin
Cultivation and Extraction of Streptomycin
The antibiotic-producing organism, Streptomyces griseus, was first cultured in starch casein broth. A loopful of the S. griseus culture was aseptically inoculated into 100 mL of sterile starch casein broth contained in a 250 mL Erlenmeyer flask. The flask was placed on a rotary shaker set at 150 – 180 rpm and incubated at 28 – 30°C for 5 – 7 days. This temperature range is favorable for actinomycete growth, while continuous agitation ensured proper aeration and nutrient mixing, which are essential for the production of secondary metabolites such as streptomycin.
At the end of incubation, the culture broth, containing both microbial biomass and soluble metabolites, was processed to separate the antibiotic. The culture was filtered through Whatman No. 1 filter paper to remove the mycelial mass, leaving a clear cell-free supernatant. This supernatant contained streptomycin and other metabolites secreted during growth.
For solvent extraction, the filtrate was transferred into a sterile separating funnel, and an equal volume of organic solvent (either ethyl acetate or n-butanol) was added. The mixture was shaken vigorously for several minutes to allow partitioning of the metabolites between the aqueous and organic phases. After settling, two distinct layers were formed: the aqueous layer and the solvent layer. The solvent phase, which preferentially extracted the antibiotic, was carefully collected. This step was repeated twice to maximize yield.
The combined solvent extracts were concentrated using a rotary evaporator under reduced pressure at 40 – 50°C, or alternatively, by gentle heating in a water bath until the solvent evaporated. This process left behind the crude streptomycin extract as a residue, which was stored in sterile vials at 4°C until further use.
Preparation of Test Plates
- Prepare Mueller-Hinton Agar (MHA) by dissolving the required amount of dehydrated medium in distilled water.
- Sterilize the medium in an autoclave at 121 °C for 15 minutes.
- Cool the medium to 45–50 °C and pour 20–25 mL into sterile Petri dishes.
- Allow the agar to solidify under aseptic conditions in a laminar airflow chamber.
Preparation of Test Organisms
- Grow overnight cultures of Escherichia coli (Gram-negative), Pseudomonas aeruginosa (Gram-negative), and Staphylococcus aureus (Gram-positive) in nutrient broth at 37°C.
- Adjust cultures to approximately 0.5 McFarland standard to standardize bacterial density.
Inoculation of Agar Plates
- Dip a sterile cotton swab into the standardized bacterial suspension.
- Remove excess inoculum by rotating the swab against the inner wall of the tube.
- Streak the agar surface in three directions to obtain a uniform lawn of growth.
- Leave plates for 10–15 minutes to allow absorption of inoculum.
Preparation of Wells and Application of Samples
- Bore uniform wells (≈6 mm diameter) into the agar using a sterile cork borer.
- Maintain equal spacing between wells and avoid penetrating the agar base.
- Label each well according to the test sample.
- Add 50–100 µL of crude streptomycin extract into designated wells using a sterile micropipette.
- Add standard streptomycin solution (positive control) to one well.
- Add the extraction solvent (ethyl acetate or n-butanol) alone as the negative control.
Incubation and Observation
- Incubate the plates upright at 37 °C for 18–24 hours.
- Examine plates for zones of inhibition around the wells.
- Measure zone diameters in millimeters using a transparent ruler or digital caliper.
- Compare inhibition zones of test samples with the positive control to assess potency.
- Confirm that the negative control shows no inhibition.
Expected results
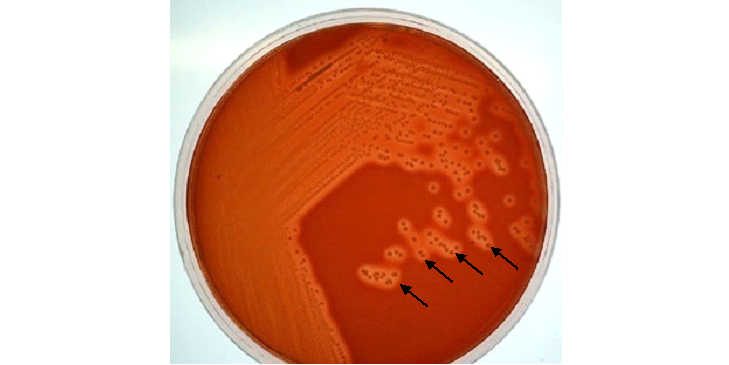
When the agar well diffusion assay is performed using the extracted streptomycin, clear zones of inhibition are expected to form around the wells containing the antibiotic extract. These zones represent areas where the growth of the test bacteria has been suppressed due to the antimicrobial action of streptomycin. The diameter of each zone serves as a direct indicator of the potency and effectiveness of the extracted compound. Larger inhibition zones typically correspond to higher activity and suggest a greater concentration or purity of the antibiotic, whereas smaller zones may indicate weaker antimicrobial effects, potentially due to lower concentrations of the active metabolite or the presence of impurities that interfere with activity.
It is also anticipated that the size of the inhibition zones will vary depending on the test organism used. Gram-negative bacteria such as Escherichia coli and Pseudomonas aeruginosa are generally more susceptible to streptomycin than many Gram-positive species. Thus, plates seeded with susceptible Gram-negative bacteria are expected to display more pronounced and wider zones of inhibition compared to those inoculated with Gram-positive strains. In addition, slight variations in zone sizes may be observed across replicates due to natural variability in diffusion rates, agar thickness, and microbial inoculum density.
Interpretation of the expected results
The presence of clear inhibition zones surrounding the wells strongly suggests that the streptomycin extract retains antimicrobial activity against the tested organisms. A comparative analysis with controls is crucial to validate these findings. The positive control, usually a commercially available antibiotic standard, is expected to produce well-defined inhibition zones. If the zones produced by the streptomycin extract are comparable in size to those of the positive control, this confirms the successful recovery of an active compound with significant antimicrobial potential. Even if the zones are slightly smaller than the control, the result remains meaningful, as it reflects that the crude extract still exhibits biologically relevant activity.
Conversely, the negative control, typically sterile solvent or culture medium without any antibiotic, should show no inhibition zones. The absence of growth inhibition in this control validates the reliability of the assay by confirming that the observed antimicrobial effect is solely due to streptomycin and not due to extraneous factors such as solvent toxicity or contamination. Together, these comparisons allow for accurate interpretation of the experiment’s outcome.
Furthermore, the spectrum of activity observed against different organisms can provide additional insights. For instance, strong activity against Gram-negative bacteria but limited effect against Gram-positive strains aligns well with the known pharmacological profile of streptomycin. Such results would reinforce the idea that the experimental extract behaves similarly to the purified commercial drug, thereby confirming both the specificity and functionality of the extraction process.
This experiment will successfully demonstrate both the extraction of streptomycin from Streptomyces griseus and the evaluation of its antimicrobial activity using the agar well diffusion method. This experiment could serve as both a practical demonstration and a reminder of the continued relevance of microbiology in safeguarding public health.
Discover more from Microbiology Class
Subscribe to get the latest posts sent to your email.